Hollow Gold-Silver Nanoshells Coated with Ultrathin SiO2 Shells for Plasmon-Enhanced Photocatalytic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Silver Nanoparticles Cores
2.3. Preparation of Hollow Gold-Silver Nanoshells (GS-NSs)
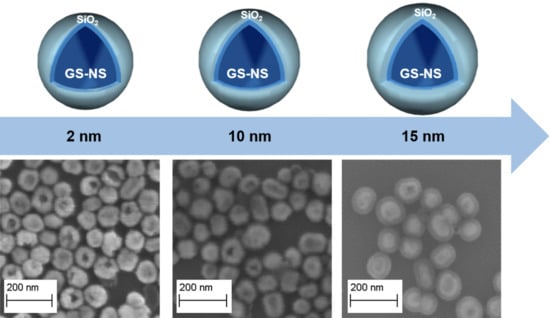
2.4. Preparation of Silica-Coated Hollow Gold-Silver Nanoshells
2.5. Characterization of SiO2-Coated Gold-Silver Nanoshells
3. Results and Discussion
3.1. Synthesis of the Hollow Gold-Silver Nanoshells
3.2. Morphology and Optical Properties of the Hollow Gold-Silver Nanoshells
3.3. Optical Properties of the Hollow Gold-Silver Nanoshells
3.4. Composition of the Hollow Gold-Silver Nanoshells
3.5. Morphology of the SiO2-Coated Gold-Silver Nanoshells
3.6. Elemental Composition of the SiO2-Coated Gold-Silver Nanoshells
3.7. Optical Properties of the SiO2-Coated Hollow Gold-Silver Nanoshells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Valenti, M.; Jonsson, M.P.; Biskos, G.; Schmidt-Ott, A.; Smith, W.A. Plasmonic Nanoparticle-Semiconductor Composites for Efficient Solar Water Splitting. J. Mater. Chem. A 2016, 4, 17891–17912. [Google Scholar]
- Tuller, H.L. Solar to Fuels Conversion Technologies: A Perspective. Mater. Renew. Sustain. Energy 2017, 6, 3. [Google Scholar] [PubMed] [Green Version]
- Izumi, Y. Recent Advances in the Photocatalytic Conversion of Carbon Dioxide to Fuels with Water and/or Hydrogen Using Solar Energy and Beyond. Coord. Chem. Rev. 2013, 257, 171–186. [Google Scholar]
- Clarizia, L.; Russo, D.; Di Somma, I.; Andreozzi, R.; Marotta, R. Hydrogen Generation through Solar Photocatalytic Processes: A Review of the Configuration and the Properties of Effective Metal-Based Semiconductor Nanomaterials. Energies 2017, 10, 1624. [Google Scholar]
- Fan, W.; Zhang, Q.; Wang, Y. Semiconductor-Based Nanocomposites for Photocatalytic H2 Production and CO2 Conversion. PCCP 2013, 15, 2632–2649. [Google Scholar] [PubMed]
- Kawasaki, S.; Takahashi, R.; Yamamoto, T.; Kobayashi, M.; Kumigashira, H.; Yoshinobu, J.; Komori, F.; Kudo, A.; Lippmaa, M. Photoelectrochemical Water Splitting Enhanced by Self-Assembled Metal Nanopillars Embedded in an Oxide Semiconductor Photoelectrode. Nat. Commun. 2016, 7, 11818. [Google Scholar]
- Concina, I.; Ibupoto, Z.H.; Vomiero, A. Semiconducting Metal Oxide Nanostructures for Water Splitting and Photovoltaics. Adv. Energy Mater. 2017, 7, 1700706. [Google Scholar]
- Medina, I.; Newton, E.; Kearney, M.R.; Mulder, R.A.; Porter, W.P.; Stuart-Fox, D. Reflection of near-Infrared Light Confers Thermal Protection in Birds. Nat. Commun. 2018, 9, 3610. [Google Scholar]
- Zhang, X.; Zhu, Y.; Yang, X.; Wang, S.; Shen, J.; Lin, B.; Li, C. Enhanced Visible Light Photocatalytic Activity of Interlayer-Isolated Triplex Ag@SiO2@TiO2 Core–Shell Nanoparticles. Nanoscale 2013, 5, 3359–3366. [Google Scholar]
- Li, J.-F.; Zhang, Y.-J.; Ding, S.-Y.; Panneerselvam, R.; Tian, Z.-Q. Core–Shell Nanoparticle-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar]
- Petryayeva, E.; Krull, U.J. Localized Surface Plasmon Resonance: Nanostructures, Bioassays and Biosensing—A Review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar]
- Acharya, D.; Mohanta, B. Optical Properties of Synthesized Ag and Ag@SiO2 Core-Shell Nanoparticles. AIP 2017, 1832, 050155. [Google Scholar]
- Li, C.-H.; Li, M.-C.; Liu, S.-P.; Jamison, A.C.; Lee, D.; Lee, T.R.; Lee, T.-C. Plasmonically Enhanced Photocatalytic Hydrogen Production from Water: The Critical Role of Tunable Surface Plasmon Resonance from Gold–Silver Nanoshells. ACS Appl. Mater. Interfaces 2016, 8, 9152–9161. [Google Scholar]
- Wang, X.; Feng, J.; Bai, Y.; Zhang, Q.; Yin, Y. Synthesis, Properties, and Applications of Hollow Micro-/Nanostructures. Chem. Rev. 2016, 116, 10983–11060. [Google Scholar]
- Li, C.-H.; Jamison, A.C.; Rittikulsittichai, S.; Lee, T.-C.; Lee, T.R. In Situ Growth of Hollow Gold–Silver Nanoshells within Porous Silica Offers Tunable Plasmonic Extinctions and Enhanced Colloidal Stability. ACS Appl. Mater. Interfaces 2014, 6, 19943–19950. [Google Scholar]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar]
- Liz-Marzán, L.M.; Giersig, M.; Mulvaney, P. Synthesis of Nanosized Gold−Silica Core−Shell Particles. Langmuir 1996, 12, 4329–4335. [Google Scholar]
- Lu, X.; Chen, J.; Skrabalak, S.E.; Xia, Y. Galvanic Replacement Reaction: A Simple and Powerful Route to Hollow and Porous Metal Nanostructures. Proc. Inst. Mech. Eng. N 2007, 221, 1–16. [Google Scholar]
- Li, H.; Xia, H.; Wang, D.; Tao, X. Simple Synthesis of Monodisperse, Quasi-Spherical, Citrate-Stabilized Silver Nanocrystals in Water. Langmuir 2013, 29, 5074–5079. [Google Scholar]
- Lee, P.C.; Meisel, D. Adsorption and Surface-Enhanced Raman of Dyes on Silver and Gold Sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Vongsavat, V.; Vittur, B.M.; Bryan, W.W.; Kim, J.-H.; Lee, T.R. Ultrasmall Hollow Gold–Silver Nanoshells with Extinctions Strongly Red-Shifted to the Near-Infrared. ACS Appl. Mater. Interfaces 2011, 3, 3616–3624. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Rusakova, I.; Hoffman, D.M.; Jacobson, A.J.; Lee, T.R. Monodisperse SnO2-Coated Gold Nanoparticles Are Markedly More Stable Than Analogous SiO2-Coated Gold Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 2479–2484. [Google Scholar] [CrossRef] [PubMed]
- Muller, O.; Dengler, S.; Ritt, G.; Eberle, B. Size and Shape Effects on the Nonlinear Optical Behavior of Silver Nanoparticles for Power Limiters. Appl. Opt. 2013, 52, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Martínez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martín Rodríguez, E.; García Solé, J. Nanoparticles for Photothermal Therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Yu, L.; Li, N. Noble Metal Nanoparticles-Based Colorimetric Biosensor for Visual Quantification: A Mini Review. Chemosensors 2019, 7, 53. [Google Scholar] [CrossRef] [Green Version]






| Nanoparticles | APTMS | Na2SiO3 | Silica Shell Thickness (nm) | |
|---|---|---|---|---|
| Concentration (µM) | Volume (mL) | Volume (µL) | ||
| 2.5 mL GS-NS (500) | 0.6 | 1.0 | 10 | 2.1 ± 1.0 |
| 1.0 | 1.0 | 25 | 11.5 ± 1.7 | |
| 1.5 | 1.0 | 35 | 15.8 ± 1.0 | |
| 2.5 mL GS-NS (700) | 0.6 | 1.0 | 10 | 1.9 ± 1.0 |
| 1.0 | 1.0 | 25 | 9.5 ± 0.9 | |
| 1.5 | 1.0 | 35 | 16.0 ± 2.7 | |
| 2.5 mL GS-NS (900) | 0.6 | 1.0 | 10 | 2.2 ± 1.3 |
| 1.0 | 1.0 | 25 | 9.8 ± 0.8 | |
| 1.5 | 1.0 | 35 | 15.1 ± 1.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinoi, P.; Marquez, M.D.; Lee, T.-C.; Lee, T.R. Hollow Gold-Silver Nanoshells Coated with Ultrathin SiO2 Shells for Plasmon-Enhanced Photocatalytic Applications. Materials 2020, 13, 4967. https://doi.org/10.3390/ma13214967
Srinoi P, Marquez MD, Lee T-C, Lee TR. Hollow Gold-Silver Nanoshells Coated with Ultrathin SiO2 Shells for Plasmon-Enhanced Photocatalytic Applications. Materials. 2020; 13(21):4967. https://doi.org/10.3390/ma13214967
Chicago/Turabian StyleSrinoi, Pannaree, Maria D. Marquez, Tai-Chou Lee, and T. Randall Lee. 2020. "Hollow Gold-Silver Nanoshells Coated with Ultrathin SiO2 Shells for Plasmon-Enhanced Photocatalytic Applications" Materials 13, no. 21: 4967. https://doi.org/10.3390/ma13214967