Preparation of Photoactive Transition-Metal Layered Double Hydroxides (LDH) to Replace Dye-Sensitized Materials in Solar Cells
Abstract
:1. Introduction
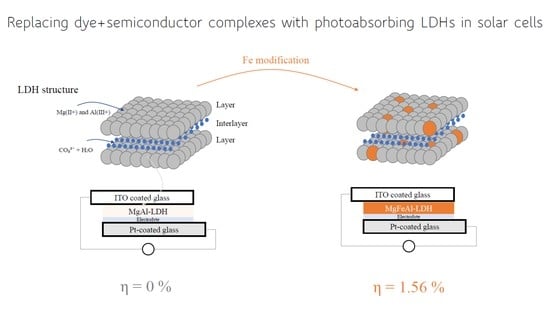
- A first reference cell containing only MgAl-LDH to determine whether this material on its own could function as a photoabsorber (SCN1).
- A second reference cell containing plain MgAl-LDH sensitized with dye (Coumarin 153) (SCN2).
- A third reference cell containing dye-sensitized (Coumarin 153) TiO2 (SCN3).
- A cell containing 5 mol% Fe modified MgFeAl-LDH which has been shown to absorb MgFeAl-LDH (SCN4).
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MgAl- and MgFeAl-LDHs
2.3. Preparation of the Solar Cells
2.4. Characterization Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lior, N. Energy resources and use: The present situation and possible paths to the future. Energy 2008, 33, 842–857. [Google Scholar] [CrossRef]
- Barnham, K.W.; Mazzer, M.; Clive, B. Resolving the energy crisis: Nuclear or photovoltaics? Nat. Mater. 2006, 5, 161–164. [Google Scholar] [CrossRef]
- Kamat, P.V. Meeting the Clean Energy Demand: Nanostructure Architectures for Solar Energy Conversion. J. Phys. Chem. C 2007, 111, 2834–2860. [Google Scholar] [CrossRef]
- Fraunhofer ISE - Annual Report 2019/20. Available online: https://www.ise.fraunhofer.de/ (accessed on 25 July 2020).
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W.; Dunlop, E.D. Solar cell efficiency tables (version 44). Prog. Photovoltaics: Res. Appl. 2014, 22, 701–710. [Google Scholar] [CrossRef]
- Saga, T. Advances in crystalline silicon solar cell technology for industrial mass production. NPG Asia Mater. 2010, 2, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Grätzel, M. Solar Energy Conversion by Dye-Sensitized Photovoltaic Cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M.; Gr, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Grätzel, M. Molecular Photovoltaics. Accounts Chem. Res. 2000, 33, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature. 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Venkatesan, S.; Lee, Y.-L. Nanofillers in the electrolytes of dye-sensitized solar cells – A short review. Co-ord. Chem. Rev. 2017, 353, 58–112. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Forano, C.; Costantino, U.; Prévot, V.; Gueho, C.T. Layered double hydroxides (LDH). In Developments in clay science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 745–782. [Google Scholar]
- Yu, J.; Wang, Q.; O’Hare, D.; Sun, L. Preparation of two dimensional layered double hydroxide nanosheets and their applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef] [PubMed]
- Gevers, B.R.; Naseem, S.; Sheppard, C.J.; Leuteritz, A.; Labuschagné, F.J.W.J. Modification of layered double hydroxides using first-row transition metals for superior UV-Vis-NIR absorption and the influence of the synthesis method used. 2020. Available online: https://10.26434/chemrxiv.11815443.v1. (accessed on 10 August 2020). Preprint.
- Naseem, S.; Lonkar, S.P.; Leuteritz, A.; Labuschagné, F.J.W.J. Different transition metal combinations of LDH systems and their organic modifications as UV protecting materials for polypropylene (PP). RSC Adv. 2018, 8, 29789–29796. [Google Scholar] [CrossRef] [Green Version]
- George, G.; Saravanakumar, M. Synthesising methods of layered double hydroxides and its use in the fabrication of dye Sensitised solar cell (DSSC): A short review. IOP Conf. Series: Mater. Sci. Eng. 2017, 263, 32020. [Google Scholar] [CrossRef] [Green Version]
- Bastianini, M.; Vivani, R.; Nocchetti, M.; Costenaro, D.; Bisio, C.; Oswald, F.; Meyer, T.B.; Marchese, L. Effect of iodine intercalation in nanosized layered double hydroxides for the preparation of quasi-solid electrolyte in DSSC devices. Sol. Energy 2014, 107, 692–699. [Google Scholar] [CrossRef]
- Ho, H.-W.; Cheng, W.-Y.; Lo, Y.-C.; Wei, T.-C.; Lu, S.-Y. Layered Double Hydroxides as an Effective Additive in Polymer Gelled Electrolyte based Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 17518–17525. [Google Scholar] [CrossRef]
- Wang, X.; Deng, R.; Kulkarni, S.A.; Wang, X.; Pramana, S.S.; Wong, C.C.; Grätzel, M.; Uchida, S.; Mhaisalkar, S. Investigation of the role of anions in hydrotalcite for quasi-solid state dye-sensitized solar cells application. J. Mater. Chem. A 2013, 1, 4345. [Google Scholar] [CrossRef]
- He, H.; Zhu, J.; Wang, N.; Luo, F.; Yang, K. Composite Gel Polymer Electrolytes Containing Layered Mg-Al Hydrotalcite for Quasi-Solid Dye-Sensitized Solar Cells. J. Electrochem. Soc. 2013, 161, H17–H20. [Google Scholar] [CrossRef]
- Foruzin, L.J.; Rezvani, Z.; Nejati, K. TiO2@NiAl-Layered double oxide nanocomposite: An excellent photoanode for a dye sensitized solar cell. Sol. Energy 2019, 186, 106–112. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Xiao, H.; Liu, D.; Qin, Y.; Wu, H.; Li, H.; Du, N.; Hou, W. Preparation and properties of mixed metal oxides based layered double hydroxide as anode materials for dye-sensitized solar cell. Chem. Eng. J. 2014, 250, 1–5. [Google Scholar] [CrossRef]
- Liu, S.; Liu, N.; Liu, J.; Wang, D.; Zhu, Y.; Li, H.; Du, N.; Xiao, H.; Hao, X.; Liu, J. The prospective photo anode composed of zinc tin mixed metal oxides for the dye-sensitized solar cells. Colloids Surfaces A: Physicochem. Eng. Asp. 2018, 547, 111–116. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Wang, T.; Wang, C.; Ge, Z.; Liu, J.; Hao, X.; Du, N.; Xiao, H. Preparation and photovoltaic properties of dye-sensitized solar cells based on zinc titanium mixed metal oxides. Colloids Surfaces A: Physicochem. Eng. Asp. 2019, 568, 59–65. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, J.; Haroone, M.S.; Chen, W.; Zheng, S.; Lu, J. Zinc-aluminum oxide solid solution nanosheets obtained by pyrolysis of layered double hydroxide as the photoanodes for dye-sensitized solar cells. J. Colloid Interface Sci. 2018, 515, 240–247. [Google Scholar] [CrossRef]
- Khodam, F.; Amani-Ghadim, A.; Aber, S. Preparation of CdS quantum dot sensitized solar cell based on ZnTi-layered double hydroxide photoanode to enhance photovoltaic properties. Sol. Energy 2019, 181, 325–332. [Google Scholar] [CrossRef]
- Lee, J.H.; Chang, J.; Cha, J.-H.; Jung, D.-Y.; Kim, S.S.; Kim, J.M. Anthraquinone Sulfonate Modified, Layered Double Hydroxide Nanosheets for Dye-Sensitized Solar Cells. Chem. Eur. J 2010, 16, 8296–8299. [Google Scholar] [CrossRef]
- Liu, X.; He, Y.; Zhang, G.; Wang, R.; Zhou, J.; Zhang, L.; Gu, J.; Jiao, T. Preparation and High Photocurrent Generation Enhancement of Self-Assembled Layered Double Hydroxide-Based Composite Dye Films. Langmuir 2020, 36, 7483–7493. [Google Scholar] [CrossRef]
- Schwenzer, B.; Neilson, J.R.; Sivula, K.; Woo, C.; Fréchet, J.M.J.; Morse, D.E. Nanostructured p-type cobalt layered double hydroxide/n-type polymer bulk heterojunction yields an inexpensive photovoltaic cell. Thin Solid Films 2009, 517, 5722–5727. [Google Scholar] [CrossRef]
- Gevers, B.R.; Naseem, S.; Leuteritz, A.; Labuschagné, F.J.W.J. Comparison of nano-structured transition metal modified tri-metal MgMAl–LDHs (M = Fe, Zn, Cu, Ni, Co) prepared using co-precipitation. RSC Adv. 2019, 9, 28262–28275. [Google Scholar] [CrossRef] [Green Version]
- Naseem, S.; Gevers, B.R.; Boldt, R.; Labuschagné, F.J.W.J.; Leuteritz, A. Comparison of transition metal (Fe, Co, Ni, Cu, and Zn) containing tri-metal layered double hydroxides (LDHs) prepared by urea hydrolysis. RSC Adv. 2019, 9, 3030–3040. [Google Scholar] [CrossRef] [Green Version]
- Parida, K.; Satpathy, M.; Mohapatra, L. Incorporation of Fe3+ into Mg/Al layered double hydroxide framework: Effects on textural properties and photocatalytic activity for H2 generation. J. Mater. Chem. 2012, 22, 7350–7357. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Rafalska-Łasocha, A.; Dziembaj, R. Influence of Cu, Co and Ni cations incorporated in brucite-type layers on thermal behaviour of hydrotalcites and reducibility of the derived mixed oxide systems. Thermochim. Acta 2002, 395, 225–236. [Google Scholar] [CrossRef]
- He, J.; Wei, M.; Li, B.; Kang, Y.; Evans, D.G.; Duan, X. Preparation of Layered Double Hydroxides. Springer Science and Business Media LLC, 2005; Volume 119, pp. 89–119. [Google Scholar]
- Ghobadi, N.; Moradian, R. Strong localization of the charge carriers in CdSe nanostructural films. Int. Nano Lett. 2013, 3, 47. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.-M.; Yan, H.; Wei, M. Band Structure Engineering of Transition-Metal-Based Layered Double Hydroxides toward Photocatalytic Oxygen Evolution from Water: A Theoretical–Experimental Combination Study. J. Phys. Chem. C 2017, 121, 2683–2695. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]







| ID | SCN1 | SCN2 | SCN3 | SCN4 |
|---|---|---|---|---|
| Setup |  |  |  |  |
| Detail | MgAl (2.10 mg) + electrolyte (0.0192 mL) | MgAl (2.15 mg) + dye (Coumarin 153) (0.0096 mL) + electrolyte (0.0192 mL) | TiO2 (2.15 mg) + dye (Coumarin 153) (0.0096 mL) + electrolyte (0.0192 mL) | MgFeAl (2.21 mg) + electrolyte (0.0192 mL) |
| FF | VOC (mV) | ISC (mA) | η (%) | |
|---|---|---|---|---|
| SCN2 | 0.945 | 69 | 0.00182 | 0.0009 |
| SCN3 | 0.941 | 81 | 0.002 | 0.0012 |
| SCN4 | 0.727 | 726 | 0.371 | 1.56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naseem, S.; Gevers, B.R.; Labuschagné, F.J.W.J.; Leuteritz, A. Preparation of Photoactive Transition-Metal Layered Double Hydroxides (LDH) to Replace Dye-Sensitized Materials in Solar Cells. Materials 2020, 13, 4384. https://doi.org/10.3390/ma13194384
Naseem S, Gevers BR, Labuschagné FJWJ, Leuteritz A. Preparation of Photoactive Transition-Metal Layered Double Hydroxides (LDH) to Replace Dye-Sensitized Materials in Solar Cells. Materials. 2020; 13(19):4384. https://doi.org/10.3390/ma13194384
Chicago/Turabian StyleNaseem, Sajid, Bianca R. Gevers, Frederick J. W. J. Labuschagné, and Andreas Leuteritz. 2020. "Preparation of Photoactive Transition-Metal Layered Double Hydroxides (LDH) to Replace Dye-Sensitized Materials in Solar Cells" Materials 13, no. 19: 4384. https://doi.org/10.3390/ma13194384