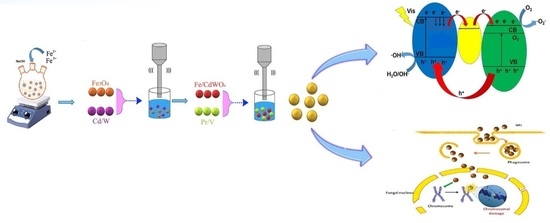
Preparation and Characterization of Magnetic Fe3O4/CdWO4 and Fe3O4/CdWO4/PrVO4 Nanoparticles and Investigation of Their Photocatalytic and Anticancer Properties on PANC1 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of Fe3O4 Nanoparticles
2.1.2. Preparation of Fe3O4/CdWO4 Nanoparticles
2.1.3. Preparation of Fe3O4/CdWO4/PrVO4 Nanoparticles
2.2. Methods
2.2.1. Assessment of Photocatalytic Performance
2.2.2. Photodegradation Mechanism
2.2.3. Cell Culture
2.2.4. MTT Assay
3. Results and Discussion
3.1. Characterization of Synthesized Nanostructures
3.2. Photocatalytic Performance
3.3. Cytotoxicity Effect on PANC1 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gutierrez, A.M.; Dziubla, T.D.; Hilt, J.Z. Recent Advances on Iron Oxide Magnetic Nanoparticles as Sorbents of Organic Pollutants in Water and Wastewater Treatment. Rev. Environ. Health 2017, 32, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Oleshkevich, E.; Teixidor, F.; Rosell, A.; Viñas, C. Merging Icosahedral Boron Clusters and Magnetic Nanoparticles: Aiming toward Multifunctional Nanohybrid Materials. Inorg. Chem. 2018, 57, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Jung, D.; Bernier, N.A.; Logan, J.K.; Waddington, M.A.; Spokoyny, A.M. Sonochemical Synthesis of Small Boron Oxide Nanoparticles. Inorg. Chem. 2018, 57, 8037–8041. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Cho, K.-Y.; Ullah, K.; Nikam, V.; Park, C.-Y.; Meng, Z.-D.; Oh, W.-C. High Photonic Effect of Organic Dye Degradation by CdSe–Graphene–TiO2 Particles. J. Ind. Eng. Chem. 2013, 19, 797–805. [Google Scholar] [CrossRef]
- Tripathy, N.; Ahmad, R.; Kuk, H.; Lee, D.H.; Hahn, Y.-B.; Khang, G. Rapid Methyl Orange Degradation Using Porous ZnO Spheres Photocatalyst. J. Photochem. Photobiol. B Biol. 2016, 161, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Cai, Y.; Zhu, X.; Han, Q.; Zhang, T.; Liu, Y.; Li, Y.; Wang, A. A Novel Photocatalytic Membrane Decorated with PDA/RGO/Ag3PO4 for Catalytic Dye Decomposition. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 68–76. [Google Scholar] [CrossRef]
- Peymani-Motlagh, S.M.; Sobhani-Nasab, A.; Rostami, M.; Sobati, H.; Eghbali-Arani, M.; Fasihi-Ramandi, M.; Ganjali, M.R.; Rahimi-Nasrabadi, M. Assessing the Magnetic, Cytotoxic and Photocatalytic Influence of Incorporating Yb3+ or Pr3+ Ions in Cobalt–Nickel Ferrite. J. Mater. Sci. Mater. Electron. 2019, 30, 6902–6909. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Ganjali, M.R.; Norouzi, P.; Faridbod, F.; Karimi, M.S. Statistically Optimized Synthesis of Dyspersium Tungstate Nanoparticles as Photocatalyst. J. Mater. Sci. Mater. Electron. 2016, 27, 12860–12868. [Google Scholar] [CrossRef]
- Hosseinpour-Mashkani, S.M.; Sobhani-Nasab, A. A Simple Sonochemical Synthesis and Characterization of CdWO4 Nanoparticles and Its Photocatalytic Application. J. Mater. Sci. Mater. Electron. 2016, 27, 3240–3244. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Aghazadeh, M.; Ganjali, M.R.; Karimi, M.S.; Novrouzi, P. Optimizing the Procedure for the Synthesis of Nanoscale Gadolinium(III) Tungstate as Efficient Photocatalyst. J. Mater. Sci. Mater. Electron. 2017, 28, 3780–3788. [Google Scholar] [CrossRef]
- Sobhani-Nasab, A.; Rangraz-Jeddy, M.; Avanes, A.; Salavati-Niasari, M. Novel Sol–Gel Method for Synthesis of PbTiO3 and Its Light Harvesting Applications. J. Mater. Sci. Mater. Electron. 2015, 26, 9552–9560. [Google Scholar] [CrossRef]
- Ahmadi, F.; Rahimi-Nasrabadi, M.; Behpour, M. Synthesis Nd2TiO5 Nanoparticles with Different Morphologies by Novel Approach and Its Photocatalyst Application. J. Mater. Sci. Mater. Electron. 2017, 28, 1531–1536. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Soofivand, F.; Sobhani-Nasab, A.; Shakouri-Arani, M.; Hamadanian, M.; Bagheri, S. Facile Synthesis and Characterization of CdTiO3 Nanoparticles by Pechini Sol–Gel Method. J. Mater. Sci. Mater. Electron. 2017, 28, 14965–14973. [Google Scholar] [CrossRef]
- Rostami, M.; Rahimi-Nasrabadi, M.; Ganjali, M.R.; Ahmadi, F.; Shojaei, A.F.; Delavar Rafiee, M. Facile Synthesis and Characterization of TiO2–Graphene–ZnFe2−x Tb x O4 Ternary Nano-Hybrids. J. Mater. Sci. 2017, 52, 7008–7016. [Google Scholar] [CrossRef]
- Eghbali-Arani, M.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Pourmasoud, S. Green Synthesis and Characterization of SmVO4 Nanoparticles in the Presence of Carbohydrates As Capping Agents with Investigation of Visible-Light Photocatalytic Properties. J. Electron. Mater. 2018, 47, 3757–3769. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Ahmadi, F.; Eghbali-Arani, M. Simple Morphology-Controlled Fabrication of CdTiO3 Nanoparticles with the Aid of Different Capping Agents. J. Mater. Sci. Mater. Electron. 2016, 27, 13294–13299. [Google Scholar] [CrossRef]
- Kooshki, H.; Sobhani-Nasab, A.; Eghbali-Arani, M.; Ahmadi, F.; Ameri, V.; Rahimi-Nasrabadi, M. Eco-Friendly Synthesis of PbTiO3 Nanoparticles and PbTiO3/Carbon Quantum Dots Binary Nano-Hybrids for Enhanced Photocatalytic Performance under Visible Light. Sep. Purif. Technol. 2019, 211, 873–881. [Google Scholar] [CrossRef]
- Sobhani-Nasab, A.; Pourmasoud, S.; Ahmadi, F.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Rahimi-Nasrabadi, M. Synthesis and Characterization of MnWO4/TmVO4 Ternary Nano-Hybrids by an Ultrasonic Method for Enhanced Photocatalytic Activity in the Degradation of Organic Dyes. Mater. Lett. 2019, 238, 159–162. [Google Scholar] [CrossRef]
- Sobhani-Nasab, A.; Behpour, M.; Rahimi-Nasrabadi, M.; Ahmadi, F.; Pourmasoud, S. New Method for Synthesis of BaFe12O19/Sm2Ti2O7 and BaFe12O19/Sm2Ti2O7/Ag Nano-Hybrid and Investigation of Optical and Photocatalytic Properties. J. Mater. Sci. Mater. Electron. 2019, 30, 5854–5865. [Google Scholar] [CrossRef]
- Wang, F.M.; Li, B.H.; Luo, Z.D.; Liu, J.Q.; Sakiyama, H.; Ma, A.Q. Magnetism and Photocatalytic Degradation of Organic Dyes Based on a New Metal Formate Framework. Russ. J. Coord. Chem. 2018, 44, 415–420. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Behpour, M.; Sobhani-Nasab, A.; Jeddy, M.R. Nanocrystalline Ce-Doped Copper Ferrite: Synthesis, Characterization, and Its Photocatalyst Application. J. Mater. Sci. Mater. Electron. 2016, 27, 11691–11697. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Behpour, M.; Sobhani-Nasab, A.; Mostafa Hosseinpour-Mashkani, S. ZnFe2−xLaxO4 Nanostructure: Synthesis, Characterization, and Its Magnetic Properties. J. Mater. Sci. Mater. Electron. 2015, 26, 9776–9781. [Google Scholar] [CrossRef]
- Wong, C.C.; Chu, W. The Hydrogen Peroxide-Assisted Photocatalytic Degradation of Alachlor in TiO2 Suspensions. Environ. Sci. Technol. 2003, 37, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Juang, R.-S. Photocatalytic Degradation of P-Chlorophenol by Hybrid H2O2 and TiO2 in Aqueous Suspensions under UV Irradiation. J. Environ. Manag. 2015, 147, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.E.; Kuo, D.-H.; Zeleke, M.A.; Zelekew, O.A.; Abay, A.K. Synthesis of Sn-WO3/g-C3N4 Composites with Surface Activated Oxygen for Visible Light Degradation of Dyes. J. Photochem. Photobiol. A Chem. 2019, 369, 133–141. [Google Scholar] [CrossRef]
- Yemmireddy, V.K.; Hung, Y.-C. Using Photocatalyst Metal Oxides as Antimicrobial Surface Coatings to Ensure Food Safety-Opportunities and Challenges: Photocatalytic Antimicrobial Coatings. Compr. Rev. Food Sci. Food Safety 2017, 16, 617–631. [Google Scholar] [CrossRef]
- Hosseinpour-Mashkani, S.S.; Sobhani-Nasab, A. Investigation the Effect of Temperature and Polymeric Capping Agents on the Size and Photocatalytic Properties of NdVO4 Nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 16459–16466. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, G.; Dong, F.; Tang, Z. The Remarkable Promotional Effect of Sn on CeVO4 Catalyst for Wide Temperature NH3 -SCR Process by Citric Acid-Assisted Solvothermal Synthesis and Post-Hydrothermal Treatment. Catal. Sci. Technol. 2018, 8, 5604–5615. [Google Scholar] [CrossRef]
- Ambard, C.; Duée, N.; Pereira, F.; Portehault, D.; Méthivier, C.; Pradier, C.-M.; Sanchez, C. Improvements in Photostability and Sensing Properties of EuVO4 Nanoparticles by Microwave-Assisted Sol–Gel Route for Detection of H2O2 Vapors. J. Sol Gel Sci. Technol. 2016, 79, 381–388. [Google Scholar] [CrossRef]
- Basu, K.; Benetti, D.; Zhao, H.; Jin, L.; Vetrone, F.; Vomiero, A.; Rosei, F. Enhanced Photovoltaic Properties in Dye Sensitized Solar Cells by Surface Treatment of SnO2 Photoanodes. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, Y.; Wu, H.; Zhou, M.; Lin, T. SmVO 4 Nanocrystals with Dodecahedral Shape: Controlled Synthesis, Growth Mechanism and Photoluminescent Properties. Mater. Res. Bull. 2018, 97, 81–88. [Google Scholar] [CrossRef]
- Adib, K.; Rezvani, Z.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M. Statistically Optimized Synthesis of Cadmium Tungstate Nanoplates for Use as a Photocatalyst. J. Mater. Sci. Mater. Electron. 2018, 29, 6377–6387. [Google Scholar] [CrossRef]
- Aslam, I.; Cao, C.; Tanveer, M.; Farooq, M.H.; Khan, W.S.; Tahir, M.; Idrees, F.; Khalid, S. A Novel Z-Scheme WO3/CdWO4 Photocatalyst with Enhanced Visible-Light Photocatalytic Activity for the Degradation of Organic Pollutants. RSC Adv. 2015, 5, 6019–6026. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Zhang, K.; Li, X.; Leng, Q.; Hu, C. Photocatalytic Activity of ZnWO4: Band Structure, Morphology and Surface Modification. ACS Appl. Mater. Interfaces 2014, 6, 14423–14432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guan, X.; Li, L.; Lin, H.; Wang, X.; Li, G. Solvent-Driven Polymorphic Control of CdWO4 Nanocrystals for Photocatalytic Performances. New J. Chem. 2012, 36, 1852. [Google Scholar] [CrossRef]
- Priya, A.M.; Selvan, R.K.; Senthilkumar, B.; Satheeshkumar, M.K.; Sanjeeviraja, C. Synthesis and Characterization of CdWO4 Nanocrystals. Ceram. Int. 2011, 37, 2485–2488. [Google Scholar] [CrossRef]
- Maavia, A.; Aslam, I.; Tanveer, M.; Rizwan, M.; Iqbal, M.W.; Tahir, M.; Hussain, H.; Boddula, R.; Yousuf, M. Facile Synthesis of G-C3N4/CdWO4 with Excellent Photocatalytic Performance for the Degradation of Minocycline. Mater. Sci. Energy Technol. 2019, 2, 258–266. [Google Scholar] [CrossRef]
- Malik, V.; Pokhriyal, M.; Uma, S. Single Step Hydrothermal Synthesis of Beyerite, CaBi2O2(CO3)2 for the Fabrication of UV-Visible Light Photocatalyst BiOI/CaBi2O2(CO3)2. RSC Adv. 2016, 6, 38252–38262. [Google Scholar] [CrossRef]
- Bhushan, M.; Kumar, Y.; Periyasamy, L.; Viswanath, A.K. Fabrication and a Detailed Study of Antibacterial Properties of α-Fe2O3/NiO Nanocomposites along with Their Structural, Optical, Thermal, Magnetic and Cytotoxic Features. Nanotechnology 2019, 30, 185101. [Google Scholar] [CrossRef]
- Lei, M.; Fu, C.; Cheng, X.; Fu, B.; Wu, N.; Zhang, Q.; Fu, A.; Cheng, J.; Gao, J.; Zhao, Z. Activated Surface Charge-Reversal Manganese Oxide Nanocubes with High Surface-to-Volume Ratio for Accurate Magnetic Resonance Tumor Imaging. Adv. Funct. Mater. 2017, 27, 1700978. [Google Scholar] [CrossRef]
- Bogdan, J.; Pławińska-Czarnak, J.; Zarzyńska, J. Nanoparticles of Titanium and Zinc Oxides as Novel Agents in Tumor Treatment: A Review. Nanoscale Res. Lett. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Çeşmeli, S.; Biray Avci, C. Application of Titanium Dioxide (TiO2 ) Nanoparticles in Cancer Therapies. J. Drug Target. 2019, 27, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, H.; Wang, S.; Sun, X.; Wang, L.; Wang, W.; Shen, H.; Liu, H. Sonodynamic Therapy (SDT): A Novel Strategy for Cancer Nanotheranostics. Sci. China Life Sci. 2018, 61, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, R.; Ouyang, J.; Chen, B. A New Strategy for TiO2 Whiskers Mediated Multi-Mode Cancer Treatment. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Seidl, C.; Ungelenk, J.; Zittel, E.; Bergfeldt, T.; Sleeman, J.P.; Schepers, U.; Feldmann, C. Tin Tungstate Nanoparticles: A Photosensitizer for Photodynamic Tumor Therapy. ACS Nano 2016, 10, 3149–3157. [Google Scholar] [CrossRef]
- AbuMousa, R.A.; Baig, U.; Gondal, M.A.; AlSalhi, M.S.; Alqahtani, F.Y.; Akhtar, S.; Aleanizy, F.S.; Dastageer, M.A. Photo-Catalytic Killing of HeLa Cancer Cells Using Facile Synthesized Pure and Ag Loaded WO3 Nanoparticles. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Han, B.; Popov, A.L.; Shekunova, T.O.; Kozlov, D.A.; Ivanova, O.S.; Rumyantsev, A.A.; Shcherbakov, A.B.; Popova, N.R.; Baranchikov, A.E.; Ivanov, V.K. Highly Crystalline WO3 Nanoparticles Are Nontoxic to Stem Cells and Cancer Cells. J. Nanomater. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Laulicht-Glick, F.; Wu, F.; Zhang, X.; Jordan, A.; Brocato, J.; Kluz, T.; Sun, H.; Costa, M. Tungsten Exposure Causes a Selective Loss of Histone Demethylase Protein. Mol. Carcinog. 2017, 56, 1778–1788. [Google Scholar] [CrossRef]
- Hariani, P.L.; Faizal, M.; Ridwan, R.; Marsi, M.; Setiabudidaya, D. Synthesis and Properties of Fe3O4 Nanoparticles by Co-Precipitation Method to Removal Procion Dye. Int. J. Environ. Sci. Developm. 2013, 336–340. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, A.; He, Q.; Chen, P.; Wei, Y.; Chen, X.; Hu, H.; Wang, M.; Huang, H.; Wang, R. Multifunctional Fe3O4@mTiO2@noble Metal Composite NPs as Ultrasensitive SERS Substrates for Trace Detection. Arab. J. Chem. 2019, in press. [Google Scholar] [CrossRef]
- Yang, S.; Zeng, T.; Li, Y.; Liu, J.; Chen, Q.; Zhou, J.; Ye, Y.; Tang, B. Preparation of Graphene Oxide Decorated Fe3O4 @SiO2 Nanocomposites with Superior Adsorption Capacity and SERS Detection for Organic Dyes. J. Nanomater. 2015, 2015, 1–8. [Google Scholar]
- Huang, G.; Zhu, Y. Synthesis and Photocatalytic Performance of ZnWO4 Catalyst. Mater. Sci. Eng. B 2007, 139, 201–208. [Google Scholar] [CrossRef]
- Yan, T.; Li, L.; Tong, W.; Zheng, J.; Wang, Y.; Li, G. CdWO4 Polymorphs: Selective Preparation, Electronic Structures, and Photocatalytic Activities. J. Solid State Chem. 2011, 184, 357–364. [Google Scholar] [CrossRef]
- Thirumalai, J.; Chandramohan, R.; Vijayan, T.A. A Novel 3D Nanoarchitecture of PrVO4 Phosphor: Selective Synthesis, Characterization, and Luminescence Behavior. Mater. Chem. Phys. 2011, 127, 259–264. [Google Scholar] [CrossRef]
- Cao, J.; Xu, B.; Luo, B.; Lin, H.; Chen, S. Novel BiOI/BiOBr Heterojunction Photocatalysts with Enhanced Visible Light Photocatalytic Properties. Catal. Commun. 2011, 13, 63–68. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Li, C.; Qiao, G.; Farooqi, A.A.; Gedanken, A.; Liu, X.; Lin, X. Zinc-Doped Copper Oxide Nanocomposites Inhibit the Growth of Pancreatic Cancer by Inducing Autophagy Through AMPK/MTOR Pathway. Front. Pharmacol. 2019, 10, 319. [Google Scholar] [CrossRef] [PubMed]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsooli, M.A.; Fasihi-Ramandi, M.; Adib, K.; Pourmasoud, S.; Ahmadi, F.; Ganjali, M.R.; Sobhani Nasab, A.; Rahimi Nasrabadi, M.; Plonska-Brzezinska, M.E. Preparation and Characterization of Magnetic Fe3O4/CdWO4 and Fe3O4/CdWO4/PrVO4 Nanoparticles and Investigation of Their Photocatalytic and Anticancer Properties on PANC1 Cells. Materials 2019, 12, 3274. https://doi.org/10.3390/ma12193274
Marsooli MA, Fasihi-Ramandi M, Adib K, Pourmasoud S, Ahmadi F, Ganjali MR, Sobhani Nasab A, Rahimi Nasrabadi M, Plonska-Brzezinska ME. Preparation and Characterization of Magnetic Fe3O4/CdWO4 and Fe3O4/CdWO4/PrVO4 Nanoparticles and Investigation of Their Photocatalytic and Anticancer Properties on PANC1 Cells. Materials. 2019; 12(19):3274. https://doi.org/10.3390/ma12193274
Chicago/Turabian StyleMarsooli, Mohammad Amin, Mahdi Fasihi-Ramandi, Kourosh Adib, Saeid Pourmasoud, Farhad Ahmadi, Mohammad Reza Ganjali, Ali Sobhani Nasab, Mahdi Rahimi Nasrabadi, and Marta E. Plonska-Brzezinska. 2019. "Preparation and Characterization of Magnetic Fe3O4/CdWO4 and Fe3O4/CdWO4/PrVO4 Nanoparticles and Investigation of Their Photocatalytic and Anticancer Properties on PANC1 Cells" Materials 12, no. 19: 3274. https://doi.org/10.3390/ma12193274