Effect of Copper Tailing Content on Corrosion Resistance of Steel Reinforcement in a Salt Lake Environment
Abstract
:1. Introduction
2. Test
2.1. Raw Materials
2.2. Test Scheme
2.3. Test Method
Electrochemical Testing Methods
3. Results and Discussion
3.1. Polarization Curve Results and Analysis
3.2. Macroscopic Morphology of Concrete
3.3. Reinforcement Micro-Structure
4. Conclusions
- The corrosion potential exhibited a negative trend with the extension of the accelerated constant-current corrosion, and the polarization curve passivation area gradually narrowed. At the same corrosion time, the corrosion potential decreases first and then increases with the increase in the copper tailings powder content. When the content of copper tailing powder is less than 20% of the total cement mass, the inhibition effect on the steel corrosion is better.
- The crack width of the reinforced concrete with copper tailing powder increased with the increase in the constant-current accelerated corrosion time. The reinforced concrete with copper tailing powder reached the failure limit, and the crack width first decreased and then increased with the increase in the copper tailing powder content. When the copper tailing content is less than 30%, the crack width was the smallest with 20% copper tailing content of cement quality.
- The corrosion current density and corrosion degree of the reinforced concrete specimens with copper tailing powder increase with the increase in electrification time. After 412 h of constant-current accelerated corrosion, the degree of corrosion of the steel reinforcement in the specimens first decrease then increase with the increase of copper tailing powder content. The degree of corrosion of the steel reinforcement was the lowest when the copper tailing content was 20%.
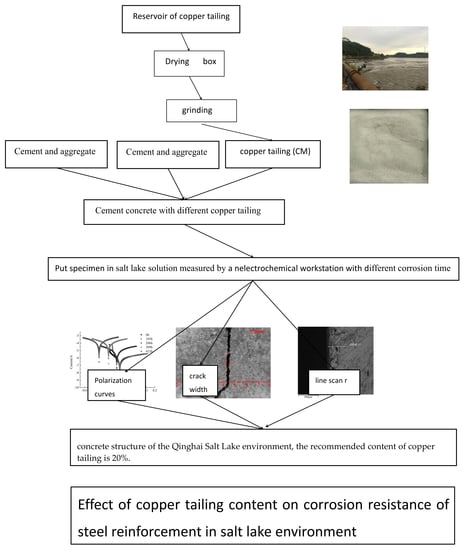
- In the reinforced concrete structure of the Qinghai Salt Lake environment, the recommended content of copper tailings is 20%.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, W.M.; Nie, W.; Zhou, G.; Zuo, Q.M. Study of dust suppression by atomized water from high pressure sprays in mines. J. Chin. Univ. Min. Technol. 2011, 40, 185–189. [Google Scholar]
- Yu, L.H.; Jia, W.L.; Xue, Y.Z. Survey and analysis of the Cooper tailing resources in China. Metal Mine 2009, 39, 179–181. (In Chinese) [Google Scholar]
- Li, H.G. Study on the influence of rain fall factors on tailings dam break and its safety warning technology. Ph.D. Thesis, University of Science & Technology Beijing, Beijing, China, April 2017. (In Chinese). [Google Scholar]
- Zheng, J.; Zhu, Y.; Zhao, Z. Utilization of limestone powder and water-reducing admixture in cemented paste backfill of coarse copper mine tailings. Constr. Build. Mater. 2016, 124, 31–36. [Google Scholar] [CrossRef]
- Rahul, S.; Rizwan, A.K. Durability assessment of self compacting concrete incorporating copper slag as fine aggregates. Constr. Build. Mater. 2017, 155, 617–629. [Google Scholar]
- Mavroulidou, M. Mechanical properties and durability of concrete with water cooled copper slag aggregate. Waste Biomass Valori. 2017, 8, 1841–1854. [Google Scholar] [CrossRef]
- Kundu, S.; Aggarwal, A.; Mazumdar, S. Stabilization characteristics of copper mine tailings through its utilization as a partial substitute for cement in concrete: Preliminary investigations. Environ. Earth. Sci. 2016, 75, 226–235. [Google Scholar] [CrossRef]
- Onuaguluchi, O.; Eren, Ö. Strength and durability properties of mortars containing copper tailings as a cement replacement material. Eur. J. Environ. Civil. Eng. 2013, 17, 19–31. [Google Scholar] [CrossRef]
- Mirhosseini, S.R.; Fadaee, M.; Tabatabaei, R.; Fadaee, M.J. Mechanical properties of concrete with Sarcheshmeh mineral complex copper slag as a part of cementitious materials. Constr. Build. Mater. 2017, 134, 44–49. [Google Scholar] [CrossRef]
- Onuaguluchi, O.; Eren, O. Copper tailings as a potential additive in concrete: Consistency, strength and toxic metal immobilization properties. Indian J. Eng. Mater. Sci. 2012, 19, 79–86. [Google Scholar]
- Fang, Y.H.; Gu, Y.M.; Kang, Q.B.; Wen, Q.; Dai, P. Utilization of copper tailing for autoclaved sand-lime brick. Constr. Build. Mater. 2011, 25, 867–872. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L.Y. Production of eco-friendly bricks from copper mine tailings through geopolymerization. Constr. Build. Mater. 2012, 29, 323–331. [Google Scholar] [CrossRef]
- Ahmari, S.; Parameswaran, K.; Zhang, L.Y. Alkali activation of copper mine tailings and low-calcium flash-furnace copper smelter slag. J. Mater. Civ. Eng. 2014, 27, 1–11. [Google Scholar] [CrossRef]
- Zhang, L.M.; Yu, H.F.; He, Z.M. Chloride ion diffusivity of salt lake concrete. J. Cent. South Univ. (Sci. Technol.) 2011, 42, 1752–1755. (In Chinese) [Google Scholar]
- Zhang, L.M.; Yu, H.F. Influence of dry-wet cycles on chloride diffusion coefficient. J. Hunan Univ. (Nat. Sci. Ed.) 2014, 41, 25–30. (In Chinese) [Google Scholar]
- Yu, H.F.; Sun, W.; Wang, J.C.; Yan, L.H.; Qu, W.; Wei, Z.Y. Circumstance of salt lakes and the durability of concrete or reinforced concrete. Ind. Constr. 2003, 33, 1–4. (In Chinese) [Google Scholar]
- Zhang, L.M.; Yu, H.F. Application of the grey system theory to predict the chloride ion capacity of concrete subjected to salt lake environment. In Proceedings of the 7th Grey Systems and Intelligent Services (GSIS), Stockholm, Sweden, 8–11 August 2017; pp. 219–223. [Google Scholar]
- Liu, L.X. Brief introduction on the study of erosion and prevention of concrete in salt lake and saline soil area of chaerhan, chaidamu. J. Build. Mater. 2001, 4, 395–400. [Google Scholar]
- Zheng, Y.L.; Zheng, J.Q.; Zhang, M. Experimental Study on Effect of Concrete Carbonization Degrees on Chloride Diffusion Coefficient. J. Tongji Univ. (Nat. Sci.) 2010, 38, 412–416. [Google Scholar]
- Panda, B.; Unluer, C.; Tan, M.J. Extrusion and rheology characterization of geopolymer nanocomposites used in 3D printing. Compos. Part B Eng. 2019, 176, 1072–1090. [Google Scholar] [CrossRef]
- Panda, B.; Tan, M.J. Rheological behavior of high volume fly ash mixtures containing micro silica for digital construction application. Mater. Lett. 2019, 237, 348–351. [Google Scholar] [CrossRef]
- Onuaguluchi, O.; Eren, Ö. Reusing copper tailings in concrete: Corrosion performance and socioeconomic implications for the Lefke-Xeros area of Cyprus. J. Clean. Prod. 2016, 112, 420–429. [Google Scholar] [CrossRef]
- Thomas, B.S.; Damare, A.; Gupta, R.C. Strength and durability characteristics of copper tailing concrete. Constr. Build. Mater. 2013, 48, 894–900. [Google Scholar] [CrossRef]
- Wu, F.; Gong, J.H.; Zhang, Z. Calculation of steel corrosion rate based on corrosive crack of beams. J. Build. Struct. 2013, 34, 144–150. [Google Scholar]
- Abosrra, L.; Ashour, A.F.; Youseffi, M. Corrosion of steel reinforcement in concrete of di-fferent compressive strengths. Constr. Build. Mater. 2011, 25, 3915–3925. [Google Scholar] [CrossRef]
- Ma, Y.; Che, Y.; Gong, J.X. Behavior of corrosion damaged circular reinforced concrete columns under cyclic loading. Constr. Build. Mater. 2012, 29, 548–556. [Google Scholar] [CrossRef]
- Syed Ayub, A. A prediction model for the residual flexural strength of corroded reinforced concrete beams. Master’s Thesis, King Fahd University of Petroleum and Minerals, Dhahran, Saudi Arabia, January 2005. [Google Scholar]
- Katzer, J. Median diameter as a grading characteristic for fine aggregate cement composite designing. Constr. Build. Mater. 2012, 35, 884–887. [Google Scholar] [CrossRef]
- Li, H.; Xu, D.L.; Feng, S.H.; Shang, B.M. Micro-structure and performance of fly ash micro-beads in cementitious material system. Constr. Build. Mater. 2014, 52, 422–427. [Google Scholar] [CrossRef]
- Duan, P.; Shui, Z.H.; Chen, W.; Shen, C.H. Effects of metakaolin, silica fume and slag on pore structure, interfacial transition zone and compressive strength of concrete. Constr. Build. Mater. 2013, 44, 1–6. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Zhang, B.; Yan, P.Y. Comparative study of effect of raw and densified silica fume in the paste, mortar and concrete. Constr. Build. Mater. 2016, 105, 82–93. [Google Scholar] [CrossRef]
- Han, F.H.; Wang, Q.; Feng, J.J. The differences among the roles of ground fly ash in the paste, mortar and concrete. Constr. Build. Mater. 2015, 93, 172–179. [Google Scholar]
- Stefanonia, M.; Angsta, U.; Elsener, B. Corrosion rate of carbon steel in carbonated concrete—A critical review. Cement Concrete Res. 2018, 103, 35–48. [Google Scholar] [CrossRef]
- Qiao, H.X.; Li, J.C.; Wen, S.Y.; Wang, P.H.; Guo, X.K. Experimental study on anticorrosion of coated reinforcement in magnesium oxychloride concrete. J. Funct. Mater. 2018, 49, 12137–12143. (In Chinese) [Google Scholar]
- Chen, T.; Tan, T.; Huang, W.L.; Yi, B.; Yuan, X.J.; Zhang, J.X. Polarization curve is used to measure corrosion rate and parameter optimization of galvanized parts of electric power equipment. Corros. Prot. 2014, 35, 120–123. (In Chinese) [Google Scholar]
- Sun, W.; Zhang, Y.S.; Liu, S.F.; Zhang, Y.M. The influence of mineral admixtures on resistance to corrosion of steel bars in green high-performance concrete. Cement Concrete Res. 2004, 34, 1781–1785. [Google Scholar] [CrossRef]
- Shi, J.J.; Sun, W. Effect of Mineral Admixtures and Steel Surface Conditions on Steel Corrosion in Mortar. J. Chin. Ceram. Soc. 2011, 39, 54–62. (In Chinese) [Google Scholar]










| Binder Material Type | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Loss |
|---|---|---|---|---|---|---|---|
| Cement | 66.5 | 5.5 | 3.3 | 15.7 | 1.7 | 2.0 | 5.3 |
| Copper tailing | 58.5 | 6.6 | 15.8 | 12.7 | 2.8 | 3.2 | 0.4 |
| No. | Raw Material Quality (kg/m3) | Slump/mm | 28 Day Compressive Strength/MPa | ||||
|---|---|---|---|---|---|---|---|
| Cement | Water | Sand | Coarse Aggregate | Copper Tailing | |||
| P | 450 | 158 | 634 | 1167 | 0 | 98 | 46.6 |
| P10 | 405 | 158 | 634 | 1167 | 45 | 100 | 50.3 |
| P20 | 360 | 158 | 634 | 1167 | 90 | 105 | 50.6 |
| P30 | 315 | 158 | 634 | 1167 | 135 | 118 | 45.1 |
| Ion Name | Na+ | Mg2+ | K+ | Ca2+ | Cl− | SO42− | CO32− | HCO3− |
|---|---|---|---|---|---|---|---|---|
| Unit (mg/dm3) | 68.36 | 35.13 | 5.98 | 4.24 | 204.21 | 22.29 | 0.17 | 0.13 |
| icorr/(μA·cm−2) | icorr < 0.2 | 0.2 < icorr < 0.5 | 0.5 < icorr < 1.0 | 1.0 < icorr <10 | icorr > 10 |
|---|---|---|---|---|---|
| Corrosion degree | Passivation state | Low corrosion condition | Moderate corrosion condition | High corrosion condition | Extreme corrosion condition |
| No. | t (h) | 0 | 103 | 206 | 309 | 412 |
|---|---|---|---|---|---|---|
| P | Ecorr/V | −0.241 | −0.337 | −0.474 | −0.508 | −0.551 |
| icorr/(μA·cm−2) | 0.007 | 0.009 | 0.066 | 0.726 | 1.078 | |
| CR/(10−3 mm·a−1) | 0.141 | 0.616 | 3.751 | 11 | 15.47 | |
| P10 | Ecorr/V | −0.199 | −0.198 | −0.234 | −0.327 | −0.434 |
| icorr/(μA·cm−2) | 0.008 | 0.011 | 0.172 | 0.446 | 0.724 | |
| CR/(10−3 mm·a−1) | 0.098 | 0.123 | 1.995 | 5.17 | 8.393 | |
| P20 | Ecorr/V | −0.175 | −0.245 | −0.283 | −0.346 | −0.356 |
| icorr/(μA·cm−2) | 0.018 | 0.040 | 0.299 | 0.256 | 0.397 | |
| CR/(10−3 mm·a−1) | 0.209 | 0.466 | 3.466 | 2.972 | 4.6 | |
| P30 | Ecorr/V | −0.241 | −0.412 | −0.526 | −0.449 | −0.486 |
| icorr/(μA·cm−2) | 0.012 | 0.053 | 0.323 | 0.948 | 1.334 | |
| CR/(10−3 mm·a−1) | 0.081 | 0.109 | 0.771 | 8.422 | 12.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, J.; Qiao, H. Effect of Copper Tailing Content on Corrosion Resistance of Steel Reinforcement in a Salt Lake Environment. Materials 2019, 12, 3069. https://doi.org/10.3390/ma12193069
Zhang L, Li J, Qiao H. Effect of Copper Tailing Content on Corrosion Resistance of Steel Reinforcement in a Salt Lake Environment. Materials. 2019; 12(19):3069. https://doi.org/10.3390/ma12193069
Chicago/Turabian StyleZhang, Liming, Jia Li, and Hongxia Qiao. 2019. "Effect of Copper Tailing Content on Corrosion Resistance of Steel Reinforcement in a Salt Lake Environment" Materials 12, no. 19: 3069. https://doi.org/10.3390/ma12193069