Adhesive Cements That Bond Soft Tissue Ex Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Milling
2.3. Materials Characterization
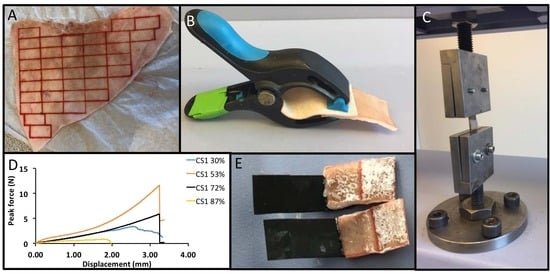
2.4. Sample Preparation
2.5. Mechanical Testing
2.6. Statistical Analysis
3. Results
3.1. Composition, Crystallinity, and Particle Size of PMC Precursors
3.2. Selection of Test Conditions
3.3. Lap Shear Strength of PMCs
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Chen, W.; Wang, R.; Xu, T.; Ma, X.; Yao, Z.; Chi, B.; Xu, H. A mussel-inspired poly (γ-glutamic acid) tissue adhesive with high wet strength for wound closure. J. Mater. Chem. B 2017, 5, 5668–5678. [Google Scholar] [CrossRef]
- Singer, A.J.; Quinn, J.V.; Hollander, J.E. The cyanoacrylate topical skin adhesives. Am. J. Emerg. Med. 2008, 26, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, A. Tissue Adhesives in Endosurgery. Semin. Laparosc. Surg. 2001, 8, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ghobril, C.; Grinstaff, M.W. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: A tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.L.; Vollenweider, L.; Xu, F.; Lee, B.P. Adhesive Performance of Biomimetic Adhesive-Coated Biologic Scaffolds. Biomacromolecules 2010, 11, 2976–2984. [Google Scholar] [CrossRef] [PubMed]
- Al-Mubarak, L.; Al-Haddab, M. Cutaneous wound closure materials: An overview and update. J. Cutan. Aesthet. Surg. 2013, 6, 178–188. [Google Scholar] [CrossRef]
- Lustgarten, L.; Abadi, J.R.; Sancevic, R.; Meneses, P.; Perez Morrel, A.; Lugo, J. Use of a protein-based tissue adhesive as an aid for the surgical reconstruction of advanced and recurrent skin cancer tumors to the head and neck region: A technical report. Surg. Neurol. 2007, 68, 53–59. [Google Scholar] [CrossRef]
- Bochyńska, A.I.; Hannink, G.; Buma, P.; Grijpma, D.W. Adhesion of tissue glues to different biological substrates. Polym. Adv. Technol. 2017, 28, 1294–1298. [Google Scholar] [CrossRef]
- Hadba, A.R.; Belcheva, N.; Jones, F.; Abuzaina, F.; Calabrese, A.; Kapiamba, M.; Skalla, W.; Taylor, J.L.; Rodeheaver, G.; Kennedy, J. Isocyanate-functional adhesives for biomedical applications. Biocompatibility and feasibility study for vascular closure applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 99, 27–35. [Google Scholar] [CrossRef]
- Sánchez-Fernández, M.J.; Hammoudeh, H.; Félix Lanao, R.P.; van Erk, M.; van Hest, J.C.M.; Leeuwenburgh, S.C.G. Bone-Adhesive Materials: Clinical Requirements, Mechanisms of Action, and Future Perspective. Adv. Mater. Interfaces 2019, 6, 1802021. [Google Scholar] [CrossRef]
- Shao, H.; Bachus, K.N.; Stewart, R.J. A water-borne adhesive modeled after the sandcastle glue of P. californica. Macromol. Biosci. 2009, 9, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bré, L.P.; Zhao, T.; Zheng, Y.; Newland, B.; Wang, W. Mussel-inspired hyperbranched poly(amino ester) polymer as strong wet tissue adhesive. Biomaterials 2014, 35, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Bré, L.P.; Zheng, Y.; Pêgo, A.P.; Wang, W. Taking tissue adhesives to the future: From traditional synthetic to new biomimetic approaches. Biomater. Sci. 2013, 1, 239–253. [Google Scholar] [CrossRef]
- Currie, L.J.; Sharpe, J.R.; Martin, R. The Use of Fibrin Glue in Skin Grafts and Tissue-Engineered Skin Replacements: A Review. Plast. Reconstr. Surg. 2001, 108, 1713–1726. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Soman, D.; Payanam, U.; Laurent, A.; Labarre, D.; Jayakrishnan, A. A novel injectable tissue adhesive based on oxidized dextran and chitosan. Acta Biomater. 2017, 53, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Serrero, A.; Trombotto, S.; Bayon, Y.; Gravagna, P.; Montanari, S.; David, L. Polysaccharide-Based Adhesive for Biomedical Applications: Correlation between Rheological Behavior and Adhesion. Biomacromolecules 2011, 12, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Spotnitz, W.D.; Burks, S. State-of-the-art review: Hemostats, sealants, and adhesives II: Update as well as how and when to use the components of the surgical toolbox. Clin. Appl. Thromb. Hemost. 2010, 16, 497–514. [Google Scholar] [CrossRef]
- Wang, G.; Liu, N.; Guo, M. Use of Whey Protein as a Natural Polymer for Tissue Adhesive: Preliminary Formulation and Evaluation In Vitro. Polymers 2018, 10, 843. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Weng, H.; Gyawali, D.; Tang, L.; Yang, J. Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure. Biomaterials 2012, 33, 7972–7983. [Google Scholar] [CrossRef]
- Sekine, T.; Nakamura, T.; Shimizu, Y.; Ueda, H.; Matsumoto, K.; Takimoto, Y.; Kiyotani, T. A new type of surgical adhesive made from porcine collagen and polyglutamic acid. J. Biomed. Mater. Res. 2001, 54, 305–310. [Google Scholar] [CrossRef]
- Bouten, P.J.M.; Zonjee, M.; Bender, J.; Yauw, S.T.K.; van Goor, H.; van Hest, J.C.M.; Hoogenboom, R. The chemistry of tissue adhesive materials. Prog. Polym. Sci. 2014, 39, 1375–1405. [Google Scholar] [CrossRef]
- Sierra, D.H. Fibrin Sealant Adhesive Systems: A Review of Their Chemistry, Material Properties and Clinical Applications. J. Biomater. Appl. 1993, 7, 309–352. [Google Scholar] [CrossRef]
- Scognamiglio, F.; Travan, A.; Rustighi, I.; Tarchi, P.; Palmisano, S.; Marsich, E.; Borgogna, M.; Donati, I.; de Manzini, N.; Paoletti, S. Adhesive and sealant interfaces for general surgery applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Assmann, A.; Vegh, A.; Ghasemi-Rad, M.; Bagherifard, S.; Cheng, G.; Sani, E.S.; Ruiz-Esparza, G.U.; Noshadi, I.; Lassaletta, A.D.; Gangadharan, S.; et al. A highly adhesive and naturally derived sealant. Biomaterials 2017, 140, 115–127. [Google Scholar] [CrossRef]
- Nakagawa, S.; Uda, H.; Sarukawa, S.; Sunaga, A.; Asahi, R.; Chi, D.; Yoshimura, K. Contact Dermatitis Caused by Dermabond Advanced Use. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1841. [Google Scholar] [CrossRef]
- Bhagat, V.; Becker, M.L. Degradable Adhesives for Surgery and Tissue Engineering. Biomacromolecules 2017, 18, 3009–3039. [Google Scholar] [CrossRef] [PubMed]
- Angulo Jerez, A.; Itxaso Sebastián Ponce, M.; José Martínez García, F.; Torregrosa Coque, R.; Martin-Martinez, J.; María Madariaga Oryan, A. Comparative Effectiveness of Cyanoacrylate Bioadhesives and Monofilament Suture in Wound Healing: A Histopathological and Physicochemical Study in New Zealand White Rabbit. J. Cytol. Histol. 2016, 7. [Google Scholar] [CrossRef]
- Ji, Y.; Ji, T.; Liang, K.; Zhu, L. Mussel-inspired soft-tissue adhesive based on poly(diol citrate) with catechol functionality. J. Mater. Sci. Mater. Med. 2016, 27, 30. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.D.; Nanci, A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: Ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc. Res. Tech. 1996, 33, 141–164. [Google Scholar] [CrossRef]
- Reinstorf, A.; Hempel, U.; Olgemöller, F.; Domaschke, H.; Schneiders, W.; Mai, R.; Stadlinger, B.; Rösen-Wolff, A.; Rammelt, S.; Gelinsky, M.; et al. O-phospho-L-serine modified calcium phosphate cements—Material properties, in vitro and in vivo investigations. Mater. Werkst. 2006, 37, 491–503. [Google Scholar] [CrossRef]
- Reinstorf, A.; Ruhnow, M.; Gelinsky, M.; Pompe, W.; Hempel, U.; Wenzel, K.W.; Simon, P. Phosphoserine—A convenient compound for modification of calcium phosphate bone cement collagen composites. J. Mater. Sci. Mater. Med. 2004, 15, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Tian, X.; Kim, J.P.; Xie, D.; Ao, X.; Shan, D.; Lin, Q.; Hudock, M.R.; Bai, X.; Yang, J. Citrate-based materials fuel human stem cells by metabonegenic regulation. Proc. Natl. Acad. Sci. USA 2018, 115, E11741–E11750. [Google Scholar] [CrossRef] [PubMed]
- Meikle, S.T.; Bianchi, G.; Olivier, G.; Santin, M. Osteoconductive phosphoserine-modified poly (ε-lysine) dendrons: Synthesis, titanium oxide surface functionalization and response of osteoblast-like cell lines. J. R. Soc. Interface 2013, 10, 20120765. [Google Scholar] [CrossRef] [PubMed]
- Merolli, A.; Santin, M. Role of Phosphatidyl-Serine in Bone Repair and Its Technological Exploitation. Molecules 2009, 14, 5367–5381. [Google Scholar] [CrossRef] [PubMed]
- Offer, L.; Veigel, B.; Pavlidis, T.; Heiss, C.; Gelinsky, M.; Reinstorf, A.; Wenisch, S.; Lips, K.S.; Schnettler, R. Phosphoserine-modified calcium phosphate cements: Bioresorption and substitution. J. Tissue Eng. Regen. Med. 2011, 5, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Chen, X.; Cheng, S.; Guo, X.; Chen, H.; Xu, H.Z. Phosphoserine promotes osteogenic differentiation of human adipose stromal cells through bone morphogenetic protein signalling. Cell Biol. Int. 2014, 38, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Raucci, M.G.; Alvarez-Perez, M.A.; Meikle, S.; Ambrosio, L.; Santin, M. Poly (Epsilon-Lysine) Dendrons Tethered with Phosphoserine Increase Mesenchymal Stem Cell Differentiation Potential of Calcium Phosphate Gels. Tissue Eng. Part A 2013, 20, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Kelly, C.; von Windheim, N.; Gall, K. Bioinspired Mineral–Organic Bioresorbable Bone Adhesive. Adv. Healthc. Mater. 2018, 7, 1800467. [Google Scholar] [CrossRef] [PubMed]
- Pujari-Palmer, M.; Guo, H.; Wenner, D.; Autefage, H.; Spicer, D.C.; Stevens, M.M.; Omar, O.; Thomsen, P.; Edén, M.; Insley, G.; et al. A Novel Class of Injectable Bioceramics That Glue Tissues and Biomaterials. Materials 2018, 11, 2492. [Google Scholar] [CrossRef] [PubMed]
- Procter, P.; Pujari-Palmer, M.; Hulsart-Billström, G.; Wenner, D.; Insley, G.; Larsson, S.; Engqvist, H. A biomechanical test model for evaluating osseous and osteochondral tissue adhesives. BMC Biomed. Eng. 2019, 1, 11. [Google Scholar] [CrossRef]
- Schneiders, W.; Reinstorf, A.; Pompe, W.; Grass, R.; Biewener, A.; Holch, M.; Zwipp, H.; Rammelt, S. Effect of modification of hydroxyapatite/collagen composites with sodium citrate, phosphoserine, phosphoserine/RGD-peptide and calcium carbonate on bone remodelling. Bone 2007, 40, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Zappone, B.; Thurner, P.J.; Adams, J.; Fantner, G.E.; Hansma, P.K. Effect of Ca2+ ions on the adhesion and mechanical properties of adsorbed layers of human osteopontin. Biophys. J. 2008, 95, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.; Nielsen, M.S.; Haselmann, K.F.; Petersen, T.E.; Sørensen, E.S. Post-translationally modified residues of native human osteopontin are located in clusters: Identification of 36 phosphorylation and five O-glycosylation sites and their biological implications. Biochem. J. 2005, 390, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Cavelier, S.; Dastjerdi, A.K.; McKee, M.D.; Barthelat, F. Bone toughness at the molecular scale: A model for fracture toughness using crosslinked osteopontin on synthetic and biogenic mineral substrates. Bone 2018, 110, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Nikel, O.; Laurencin, D.; McCallum, S.A.; Gundberg, C.M.; Vashishth, D. NMR Investigation of the Role of Osteocalcin and Osteopontin at the Organic–Inorganic Interface in Bone. Langmuir 2013, 29, 13873–13882. [Google Scholar] [CrossRef] [PubMed]
- Thurner, P.J.; Chen, C.G.; Ionova-Martin, S.; Sun, L.; Harman, A.; Porter, A.; Ager, J.W.; Ritchie, R.O.; Alliston, T. Osteopontin deficiency increases bone fragility but preserves bone mass. Bone 2010, 46, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Fantner, G.E.; Adams, J.; Turner, P.; Thurner, P.J.; Fisher, L.W.; Hansma, P.K. Nanoscale Ion Mediated Networks in Bone: Osteopontin Can Repeatedly Dissipate Large Amounts of Energy. Nano Lett. 2007, 7, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Hansma, P.K.; Fantner, G.E.; Kindt, J.H.; Thurner, P.J.; Schitter, G.; Turner, P.J.; Udwin, S.F.; Finch, M.M. Sacrificial bonds in the interfibrillar matrix of bone. J. Musculoskelet. Neuronal Interact. 2005, 5, 313–315. [Google Scholar]
- Thurner, P.J.; Lam, S.; Weaver, J.C.; Morse, D.E.; Hansma, P.K. Localization of Phosphorylated Serine, Osteopontin, and Bone Sialoprotein on Bone Fracture Surfaces. J. Adhes. 2009, 85, 526–545. [Google Scholar] [CrossRef]
- Fantner, G.E.; Rabinovych, O.; Schitter, G.; Thurner, P.; Kindt, J.H.; Finch, M.M.; Weaver, J.C.; Golde, L.S.; Morse, D.E.; Lipman, E.A.; et al. Hierarchical interconnections in the nano-composite material bone: Fibrillar cross-links resist fracture on several length scales. Compos. Sci. Technol. 2006, 66, 1205–1211. [Google Scholar] [CrossRef]
- Fantner, G.E.; Hassenkam, T.; Kindt, J.H.; Weaver, J.C.; Birkedal, H.; Pechenik, L.; Cutroni, J.A.; Cidade, G.A.G.; Stucky, G.D.; Morse, D.E.; et al. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat. Mater. 2005, 4, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Tödtmann, N.; Lode, A.; Mann, R.; Mai, R.; Lauer, G.; Wieczorek, K.; Eckelt, U. Influence of different modifications of a calcium phosphate cement on resorption and new bone formation: An in vivo study in the minipig. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Liber-Kneć, A.; Łagan, S. Factors influencing on mechanical properties of porcine skin obtained in tensile test-preliminary studies. In Proceedings of the Innovations in Biomedical Engineering, Cham, Switzerland, 24 October 2018; pp. 255–262. [Google Scholar]
- Ranamukhaarachchi, S.A.; Lehnert, S.; Ranamukhaarachchi, S.L.; Sprenger, L.; Schneider, T.; Mansoor, I.; Rai, K.; Häfeli, U.O.; Stoeber, B. A micromechanical comparison of human and porcine skin before and after preservation by freezing for medical device development. Sci. Rep. 2016, 6, 32074. [Google Scholar] [CrossRef] [PubMed]
- Sierra, D.H.; Feldman, D.S.; Saltz, R.; Huang, S. A method to determine shear adhesive strength of fibrin sealants. J. Appl. Biomater. 1992, 3, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Quinn, J.; Wells, G.; Sutcliffe, T.; Jarmuske, M.; Maw, J.; Stiell, I.; Johns, P. A Randomized Trial Comparing Octylcyanoacrylate Tissue Adhesive and Sutures in the Management of Lacerations. JAMA 1997, 277, 1527–1530. [Google Scholar] [CrossRef]
- Mai, R.; Lux, R.; Proff, P.; Lauer, G.; Pradel, W.; Leonhardt, H.; Reinstorf, A.; Gelinsky, M.; Jung, R.; Eckelt, U.; et al. O-phospho-l-serine: A modulator of bone healing in calcium-phosphate cements. Biomed. Tech. Biomed. Eng. 2008, 53, 229–233. [Google Scholar] [CrossRef]
- Mai, R.; Reinstorf, A.; Pilling, E.; Hlawitschka, M.; Jung, R.; Gelinsky, M.; Schneider, M.; Loukota, R.; Pompe, W.; Eckelt, U.; et al. Histologic study of incorporation and resorption of a bone cement—Collagen composite: An in vivo study in the minipig. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, e9–e14. [Google Scholar] [CrossRef]
- Vater, C.; Lode, A.; Bernhardt, A.; Reinstorf, A.; Nies, B.; Gelinsky, M. Modifications of a calcium phosphate cement with biomolecules—Influence on nanostructure, material, and biological properties. J. Biomed. Mater. Res. Part A 2010, 95, 912–923. [Google Scholar] [CrossRef]
- Wynn-Jones, G.; Shelton, R.M.; Hofmann, M.P. Injectable citrate-modified Portland cement for use in vertebroplasty. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1799–1808. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Pujari-Palmer, M.; Wenner, D.; Procter, P.; Insley, G.; Engqvist, H. Adhesive Cements That Bond Soft Tissue Ex Vivo. Materials 2019, 12, 2473. https://doi.org/10.3390/ma12152473
Li X, Pujari-Palmer M, Wenner D, Procter P, Insley G, Engqvist H. Adhesive Cements That Bond Soft Tissue Ex Vivo. Materials. 2019; 12(15):2473. https://doi.org/10.3390/ma12152473
Chicago/Turabian StyleLi, Xiuwen, Michael Pujari-Palmer, David Wenner, Philip Procter, Gerard Insley, and Håkan Engqvist. 2019. "Adhesive Cements That Bond Soft Tissue Ex Vivo" Materials 12, no. 15: 2473. https://doi.org/10.3390/ma12152473