Ventriculoperitoneal Shunts Equipped with On-Off Valves for Intraventricular Therapies in Patients with Communicating Hydrocephalus due to Leptomeningeal Metastases
Abstract
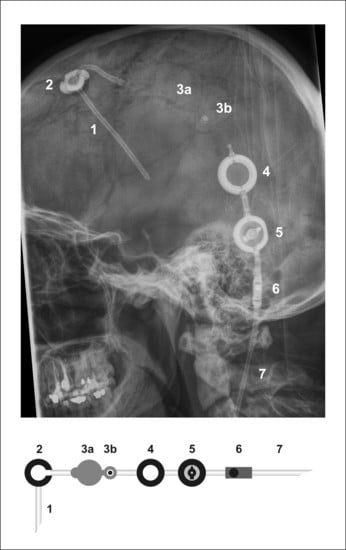
:1. Introduction
2. Results
3. Discussion
4. Patients and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Le Rhun, E.; Weller, M.; Brandsma, D.; Van den Bent, M.; de Azambuja, E.; Henriksson, R.; Boulanger, T.; Peters, S.; Watts, C.; Wick, W.; et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann. Oncol. 2017, 28, iv84–iv99. [Google Scholar] [CrossRef] [PubMed]
- Phuphanich, S.; Maria, B.; Braeckman, R.; Chamberlain, M. A pharmacokinetic study of intra-CSF administered encapsulated cytarabine (DepoCyt) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma, or solid tumors as part of a phase III study. J. Neurooncol. 2007, 81, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Glantz, M.J.; Van Horn, A.; Fisher, R.; Chamberlain, M.C. Route of intracerebrospinal fluid chemotherapy administration and efficacy of therapy in neoplastic meningitis. Cancer 2010, 116, 1947–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamberlain, M.C.; Kormanik, P.; Howell, S.B.; Kim, S. Pharmacokinetics of intralumbar DTC-101 for the treatment of leptomeningeal metastases. Arch. Neurol. 1995, 52, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chatelut, E.; Kim, J.C.; Howell, S.B.; Cates, C.; Kormanik, P.A.; Chamberlain, M.C. Extended CSF cytarabine exposure following intrathecal administration of DTC 101. J. Clin. Oncol. 1993, 11, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Moser, A.M.; Adamson, P.C.; Gillespie, A.J.; Poplack, D.G.; Balis, F.M. Intraventricular concentration times time (C × T) methotrexate and cytarabine for patients with recurrent meningeal leukemia and lymphoma. Cancer 1999, 85, 511–516. [Google Scholar] [CrossRef]
- Sandberg, D.I.; Bilsky, M.H.; Souweidane, M.M.; Bzdil, J.; Gutin, P.H. Ommaya reservoirs for the treatment of leptomeningeal metastases. Neurosurgery 2000, 47, 49–54. [Google Scholar] [PubMed]
- Volkov, A.A.; Filis, A.K.; Vrionis, F.D. Surgical treatment for leptomeningeal disease. Cancer Control 2017, 24, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zada, G.; Chen, T.C. A novel method for administering intrathecal chemotherapy in patients with leptomeningeal metastases and shunted hydrocephalus: Case report. Neurosurgery 2010, 67, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Palejwala, S.K.; Stidd, D.A.; Skoch, J.M.; Gupta, P.; Lemole, G.M., Jr.; Weinand, M.E. Use of a stop-flow programmable shunt valve to maximize CNS chemotherapy delivery in a pediatric patient with acute lymphoblastic leukemia. Surg. Neurol. Int. 2014, 5, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dunn, I.F.; Glantz, M.; Allison, D.L.; Jensen, R.; Johnson, M.D.; Friedlander, R.M.; Kesari, S. Benefit of ventriculoperitoneal cerebrospinal fluid shunting and intrathecal chemotherapy in neoplastic meningitis: A retrospective, case-controlled study. J. Neurosurg. 2011, 115, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, W.A.; Poplack, D.G. Intraventricular versus intralumbar methotrexate for central-nervous-system leukemia: Prolonged remission with the ommaya reservoir. Med. Pediatr. Oncol. 1979, 6, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C.; Kormanik, P.A.; Barba, D. Complications associated with intraventricular chemotherapy in patients with leptomeningeal metastases. J. Neurosurg. 1997, 87, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Arita, N.; Hayakawa, T.; Ushio, Y. ACNU, MTX and 5-FU penetration of rat brain tissue and tumors. J. Neurooncol. 1999, 45, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Taillibert, S.; Chamberlain, M.C. Leptomeningeal metastasis. Handb. Clin. Neurol. 2018, 149, 169–204. [Google Scholar] [PubMed]
- Grommes, C.; Oxnard, G.R.; Kris, M.G.; Miller, V.A.; Pao, W.; Holodny, A.I.; Clarke, J.L.; Lassman, A.B. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neurol. Oncol. 2011, 13, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, P.; Petrocca, F. Regional delivery of chimeric antigen receptor (CAR) T-cells for cancer therapy. Cancers 2017, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, S.S.; Jensen, M.C.; Satyamurthy, N.; Budhiraja, S.; Paik, D.; Czernin, J.; Gambhir, S.S. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat. Clin. Pract. Oncol. 2009, 6, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.R.; Bridge, C.; Murray, J.D.; Alvord, E.C., Jr. Virtual and real brain tumors: Using mathematical modeling to quantify glioma growth and invasion. J. Neurol. Sci. 2003, 216, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, U.; Forschler, H.; Kuker, W.; Meyermann, R.; Bamberg, M.; Dichgans, J.; Weller, M. Leptomeningeal metastasis: Survival and prognostic factors in 155 patients. J. Neurol. Sci. 2004, 223, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Jaeckle, K.A. Neoplastic meningitis from systemic malignancies: Diagnosis, prognosis and treatment. Semin. Oncol. 2006, 33, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C. Neoplastic meningitis. J. Clin. Oncol. 2005, 23, 3605–3613. [Google Scholar] [CrossRef] [PubMed]
- Glantz, M.J.; Jaeckle, K.A.; Chamberlain, M.C.; Phuphanich, S.; Recht, L.; Swinnen, L.J.; Maria, B.; LaFollette, S.; Schumann, G.B.; Cole, B.F.; et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin. Cancer Res. 1999, 5, 3394–3402. [Google Scholar] [PubMed]
- Wu, Y.; Green, N.L.; Wrensch, M.R.; Zhao, S.; Gupta, N. Ventriculoperitoneal shunt complications in california: 1990 to 2000. Neurosurgery 2007, 61, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Merkler, A.E.; Ch’ang, J.; Parker, W.E.; Murthy, S.B.; Kamel, H. The rate of complications after ventriculoperitoneal shunt surgery. World Neurosurg. 2017, 98, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Lara-Medina, F.; Crismatt, A.; Villarreal-Garza, C.; Alvarado-Miranda, A.; Flores-Hernandez, L.; Gonzalez-Pinedo, M.; Gamboa-Vignolle, C.; Ruiz-Gonzalez, J.D.; Arrieta, O. Clinical features and prognostic factors in patients with carcinomatous meningitis secondary to breast cancer. Breast J. 2012, 18, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Taillibert, S.; Zairi, F.; Kotecki, N.; Devos, P.; Mailliez, A.; Servent, V.; Vanlemmens, L.; Vennin, P.; Boulanger, T.; et al. A retrospective case series of 103 consecutive patients with leptomeningeal metastasis and breast cancer. J. Neurooncol. 2013, 113, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Niwinska, A.; Rudnicka, H.; Murawska, M. Breast cancer leptomeningeal metastasis: The results of combined treatment and the comparison of methotrexate and liposomal cytarabine as intra-cerebrospinal fluid chemotherapy. Clin. Breast Cancer 2015, 15, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Gwak, H.S.; Joo, J.; Kim, S.; Yoo, H.; Shin, S.H.; Han, J.Y.; Kim, H.T.; Lee, J.S.; Lee, S.H. Analysis of treatment outcomes of intraventricular chemotherapy in 105 patients for leptomeningeal carcinomatosis from non-small-cell lung cancer. J. Thorac. Oncol. 2013, 8, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Harstad, L.; Hess, K.R.; Groves, M.D. Prognostic factors and outcomes in patients with leptomeningeal melanomatosis. Neuro. Oncol. 2008, 10, 1010–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Burger, M.C.; Jennewein, L.; Genssler, S.; Schonfeld, K.; Zeiner, P.; Hattingen, E.; Harter, P.N.; Mittelbronn, M.; Tonn, T.; et al. ErbB2/HER2-specific NK cells for targeted therapy of glioblastoma. J. Natl. Cancer Inst. 2016, 108, djv375. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, P.; Romanski, A.; Miller, N.; Odendahl, M.; Bonig, H.; Zhang, C.; Seifried, E.; Wels, W.S.; Tonn, T. Clinical grade manufacturing of genetically modified, CAR-expressing NK-92 cells for the treatment of ErbB2-positive malignancies. Cancer Immunol. Immunother. 2018, 67, 25–38. [Google Scholar] [CrossRef] [PubMed]




| Pat. No. | Intrathecal Chemotherapy Applied | Reasons for Omitting Intrathecal Chemotherapy | Complications | Number of Intraventricular Injections |
|---|---|---|---|---|
| 1 | None | Nodular type of meningeal metastases | None | 0 |
| 2 | None | Nodular type of meningeal metastases | None | 0 |
| 3 | None | clinical deterioration | None | 0 |
| 4 | MTX, thiotepa | - | None | 9 MTX 17 thiotepa |
| 5 | None | nodular type of meningeal metastases | None | 0 |
| 6 | Thiotepa | - | Shunt leakage | 1 |
| Pat. No. | PFS (Weeks) | OS (Weeks) |
|---|---|---|
| 1 | 7 | 12 |
| 2 | 10 | 11 |
| 3 | 3 | 9 |
| 4 | 34 | 116 |
| 5 | 6 | 15 |
| 6 | 13 | 17 |
| Pat. No. | Age at Shunt Implantation | Sex | Underlying Disease |
|---|---|---|---|
| 1 | 33 years | F | NSCLC |
| 2 | 50 years | F | breast cancer |
| 3 | 57 years | M | transitional cell carcinoma |
| 4 | 60 years | F | NSCLC |
| 5 | 63 years | F | breast cancer |
| 6 | 70 years | M | prostate cancer |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burger, M.C.; Wagner, M.; Franz, K.; Harter, P.N.; Bähr, O.; Steinbach, J.P.; Senft, C. Ventriculoperitoneal Shunts Equipped with On-Off Valves for Intraventricular Therapies in Patients with Communicating Hydrocephalus due to Leptomeningeal Metastases. J. Clin. Med. 2018, 7, 216. https://doi.org/10.3390/jcm7080216
Burger MC, Wagner M, Franz K, Harter PN, Bähr O, Steinbach JP, Senft C. Ventriculoperitoneal Shunts Equipped with On-Off Valves for Intraventricular Therapies in Patients with Communicating Hydrocephalus due to Leptomeningeal Metastases. Journal of Clinical Medicine. 2018; 7(8):216. https://doi.org/10.3390/jcm7080216
Chicago/Turabian StyleBurger, Michael C., Marlies Wagner, Kea Franz, Patrick N. Harter, Oliver Bähr, Joachim P. Steinbach, and Christian Senft. 2018. "Ventriculoperitoneal Shunts Equipped with On-Off Valves for Intraventricular Therapies in Patients with Communicating Hydrocephalus due to Leptomeningeal Metastases" Journal of Clinical Medicine 7, no. 8: 216. https://doi.org/10.3390/jcm7080216