Differentiation of Developmental Pathways Results in Different Life-History Patterns between the High and Low Latitudinal Populations in the Asian Corn Borer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
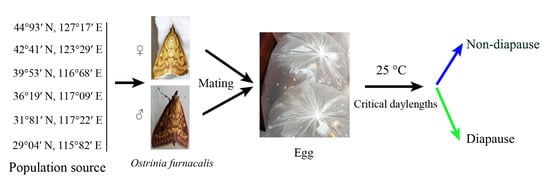
2.1. Study Populations and Insect Culture
2.2. Experimental Methods
2.3. Statistical Analyses
3. Results
3.1. Developmental Pathway and Sex Ratio in Different Geographic Populations
3.2. Developmental Time
3.3. Pupal Weight and Growth Rate
3.4. Adult Weight and Weight Loss
3.5. Sexual Size Dimorphism
3.6. Relationship between Larval Developmental Time and Pupal Weight
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lynch, M.; Gabriel, W. Environmental tolerance. Am. Nat. 1987, 129, 283–303. [Google Scholar] [CrossRef] [Green Version]
- Stearns, S.C. The evolutionary significance of phenotypic plasticity-phenotypic sources of variation among organisms can be described by developmental switches and reaction norms. Bioscience 1989, 39, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Meyers, L.A.; Bull, J.J. Fighting change with change: Adaptive variation in an uncertain world. Trends Ecol. Evol. 2002, 17, 551–557. [Google Scholar] [CrossRef]
- Liefting, M.; Hoffmann, A.A.; Ellers, J. Plasticity versus environmental canalization: Population differences in thermal responses along a latitudinal gradient in Drosophila serrata. Evolution 2009, 63, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- West-Eberhard, M.J. Developmental Plasticity and Evolution; Oxford University Press: New York, NY, USA, 2003. [Google Scholar] [CrossRef]
- Xue, F.S.; Kallenborn, H.; Wei, H.Y. Summer and winter diapause in pupae of the cabbage butterfly, Pieris melete Menetries. J. Insect Physiol. 1997, 43, 701–707. [Google Scholar] [CrossRef]
- Xue, F.S.; Kallenborn, H.G. Control of summer and winter diapause in Pidorus euchromioides (Lepidoptera; Zygaenidae) on Chinese sweetleaf Symplocs chinensis. Bull. Entomol. Res. 1998, 8, 207–211. [Google Scholar]
- Xue, F.S.; Spieth, H.R.; Li, A.Q.; Hua, A. The role of photoperiod and temperature in determination of summer and winter diapause in the cabbage beetle, Colaphellus bowringi (Coleoptera: Chrysomelidae). J. Insect Physiol. 2002, 48, 279–286. [Google Scholar] [CrossRef]
- Waldbaue, G.P. Phenological adaptation and the polymodal emergence patterns of insects. In Evolution of Insect Migration and Diapause; Dinglel, H., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1978; pp. 127–144. [Google Scholar]
- Xue, F.S.; Kallenborn, H.G. Dispersive breeding in agricultural pest insects and its adaptive significance. J. Appl. Entomol. 1993, 116, 170–177. [Google Scholar]
- Gotthard, K.; Berger, D. The diapause decision as a cascade switch for adaptive developmental plasticity in body mass in a butterfly. J. Evol. Biol. 2010, 23, 1129–1137. [Google Scholar] [CrossRef]
- Aalberg Haugen, I.M.; Berger, D.; Gotthard, K. The evolution of alternative developmental pathways: Footprints of selection on life-history traits in a butterfly. J. Evol. Biol. 2012, 25, 1377–1388. [Google Scholar] [CrossRef]
- Aalberg Haugen, I.M.; Gotthard, K. Diapause induction and relaxed selection on alternative developmental pathways in a butterfly. J. Anim. Ecol. 2015, 84, 464–472. [Google Scholar] [CrossRef]
- Masaki, S. Geographic variation and climatic adaptation in a field cricket (Orthopetera Gryllidae). Evolution 1967, 21, 725–741. [Google Scholar] [CrossRef]
- Roff, D.A. Optimizing decelopment time in a seasonal environment: The “ups and downs” of clinal variation. Oecologia 1980, 45, 202–208. [Google Scholar] [CrossRef]
- Gotthard, K.; Nylin, S.; Wiklund, C. Adaptive variation in growth rate: Life history costs and consequences in the speckled wood butterfly, Pararge aegeria. Oecologia 1994, 99, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Arnett, A.E.; Gotelli, N.J. Geographic variation in life-history traits of the ant lion, Myrmeleon immaculatus: Evolutionary implications of Bergmann’s rule. Evolution 1999, 53, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Bidau, C.J.; Martía, D.A. Dichroplus vittatus (Orthoptera: Acrididae) follows the converse to Bergmann’s rule although male morphological variability increases with latitude. Bull. Entomol. Res. 2007, 97, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Nygren, G.H.; Bergström, A.; Nylin, S. Latitudinal body size clines in the butterfly Polyommatus icarus are shaped by gene-environment interactions. J. Insect Sci. 2008, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Stillwell, R.C.; Fox, C.W. Geographic variation in body size, sexual size dimorphism and fitness components of a seed beetle: Local adaptation versus phenotypic plasticity. Oikos 2009, 118, 703–712. [Google Scholar] [CrossRef]
- Chown, S.L.; Gaston, K.J. Body size variation in insects: A macroecological perspective. Biol. Rev. Camb. Philos. Soc. 2010, 85, 139–169. [Google Scholar] [CrossRef]
- Kivelӓ, S.M.; Vӓlimӓki, P.; Carrasco, D.; Mӓenpӓӓ, M.I.; Oksanen, J. Latitudinal insect body size clines revisited: A critical evaluation of the saw-tooth model. J. Anim. Ecol. 2011, 80, 1184–1195. [Google Scholar] [CrossRef]
- Barton, M.; Sunnucks, P.; Norgate, M.; Murray, N.; Kearney, M. Co-Gradient variation in growth rate and development time of a broadly distributed butterfly. PLoS ONE 2014, 9, e95258. [Google Scholar] [CrossRef]
- Parsons, P.A.; Joern, A. Life history traits associated with body size covary along a latitudinal gradient in a generalist grasshopper. Oecologia 2014, 174, 379–391. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Roff, D.A. Adaptation to seasonality in a cricket: Patterns of phenotypic and genotypic variation in body size and diapause expression along a cline in season length. Evolution 1989, 43, 1483–1496. [Google Scholar] [CrossRef]
- Nylin, S.; Gotthard, K. Plasticity in life-history traits. Annu. Rev. Entomol. 1998, 43, 63–83. [Google Scholar] [CrossRef] [Green Version]
- Fischer, K.; Fiedler, K. Sexual differences in life-history traits in the butterfly Lycaena tityrus: A comparison between direct and diapause development. Entomol. Exp. Et Appl. 2001, 100, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.J.; He, H.M.; Wu, S.H.; Zou, C.; Xue, F.S. Expression of alternative developmental pathways in the cabbage butterfly, Pieris melete and their differences in life history traits. Ecol. Evol. 2019, 9, 12311–12321. [Google Scholar] [CrossRef] [Green Version]
- Kivelä, S.; Välimäki, P.; Mäenpää, M. Genetic and phenotypic variation in juvenile development in relation to temperature and developmental pathway in a geometrid moth. J. Evol. Biol. 2012, 25, 881–891. [Google Scholar] [CrossRef]
- Shelomi, M. Where are we now? Bergmann’s Rule Sensu Lato in Insects. Am. Nat. 2012, 180, 511–519. [Google Scholar] [CrossRef]
- Conover, D.O.; Duffy, T.A.; Hice, L.A. The covariance between genetic and environmental influences across ecological gradients: Reassessing the evolutionary significance of countergradient and cogradient variation. Ann. N. Y. Acad. Sci. 2009, 1168, 100–129. [Google Scholar] [CrossRef]
- Pöykkö, H.; Tammaru, T. Countergradient vs. cogradient variation in growth and diapause in a lichen-feeding moth, Eilema depressum (Lepidoptera: Arctiidae). J. Evol. Biol. 2010, 23, 1278–1285. [Google Scholar] [CrossRef]
- Tang, J.J.; He, H.M.; Chen, C.; Fu, S.; Xue, F.S. Latitudinal cogradient variation of development time and growth rate and a negative latitudinal body weight cline in a widely distributed cabbage beetle. PLoS ONE 2017, 12, e0181030. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; He, H.M.; Huang, L.L.; Geng, T.; Fu, S.; Xue, F.S. Variation of life-history traits of the Asian corn borer, Ostrinia furnacalis in relation to temperature and geographical latitude. Ecol. Evol. 2016, 6, 5129–5143. [Google Scholar] [CrossRef] [PubMed]
- Masaki, S. Seasonal and latitudinal adaptations in the life cycles of crickets. In Evolution of Insect Migration and Diapause; Dingle, H., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1978; pp. 72–100. [Google Scholar] [CrossRef]
- Nylin, S.; Svärd, L. Latitudinal patterns in the size of European butterflies. Holarct. Ecol. 1991, 14, 192–202. [Google Scholar] [CrossRef]
- Mousseau, T.A. Intra- and interpopulation genetic variation: Explaning the past and predicting the future. In Adaptive Genetic Variation in the Wild; Mousseau, T.A., Sinervo, B., Endler, J.A., Eds.; Oxford University Press: Oxford, UK, 2000; pp. 219–250. [Google Scholar]
- Johansson, F. Latitudinal shift in body size of Enallagma cyathigerum (Odonata). J. Biogeogr. 2003, 30, 29–34. [Google Scholar] [CrossRef]
- Du, Z.W.; Cai, W.Q. A preliminary report on photoperiodic response of the Asian corn borer, Ostrinia furnacalis (Guenée) in Jiangsu. Acta Entomol. Sin. 1964, 13, 129–132. (In Chinese) [Google Scholar]
- Gong, H.F.; Chen, P.; Wang, R.; Lian, M.L.; Xia, Z.H.; Yan, Y. The influence of photoperiod and temperature on the diapause of the Asian corn borer Ostrinia furnacalis (Guenée). Acta Entomol. Sin. 1984, 27, 280–286. (In Chinese) [Google Scholar]
- Lu, X.; Li, J.P.; Wang, Y.S. Preliminary study on voltinism types of Ostrinia furnacalis (ACB). J. Maize Sci. 1995, 3, 75–78. (In Chinese) [Google Scholar]
- Huang, L.L.; Tang, J.J.; Chen, C.; He, H.M.; Gao, Y.L.; Xue, F.S. Diapause incidence and critical day length of Asian corn borer (Ostrinia furnacalis) exhibit a latitudinal cline in both pure and hybrid strains. J. Pest Sci. 2020, 93, 559–568. [Google Scholar] [CrossRef]
- Ma, R.; Qian, H.T.; Dong, H.; Xia, X.; Cong, B. Research on the development period of over-wintering larvae of different geographic populations of Asian corn borer. Hubei Agric. Sci. 2008, 47, 541–543. (In Chinese) [Google Scholar] [CrossRef]
- Liu, N.; Wen, L.P.; He, K.L.; Wang, Z.Y.; Zhao, T.C. The cold hardiness in the different geographic populations of Asian corn borer, Ostrinia furnacalis (Guenée). Acta Phytophylacica Sin. 2005, 32, 163–168. (In Chinese) [Google Scholar] [CrossRef]
- Qiao, L.; Zheng, J.W.; Cheng, W.N.; Li, Y.P. Impact of 4 different artificial fodders on life span of Asian corn borer, Ostrinia furnacalis (Guenée). J. Northwest AF Univ. Nat. Sci. Ed. 2008, 36, 109–112. (In Chinese) [Google Scholar] [CrossRef]
- Lovich, J.E.; Gibbons, J.W. A review of techniques for quantifying sexual size dimorphism. Growth Dev. Aging 1992, 56, 269–281. [Google Scholar]
- Tauber, M.J.; Tauber, C.A.; Masaki, S. Seasonal Adaptations of Insects; Oxford University Press: New York, NY, USA, 1986. [Google Scholar]
- Blanckenhorn, W.U.; Fairbairrn, D.J. Life history adaptation along a latitudinal cline in the water strider Aquarius remigis (Heteroptera: Gerridae). J. Evol. Biol. 1995, 8, 21–41. [Google Scholar] [CrossRef]
- Burke, S.; Pullin, A.S.; Wilson, R.J.; Thomas, C.D. Selection for discontinuous life-history traits along a continuous thermal gradient in the butterfly Aricia agestis. Ecol. Entomol. 2005, 30, 613–619. [Google Scholar] [CrossRef]
- Paolucci, S.; van de Zande, L.; Beukeboom, L.W. Adaptive latitudinal cline of photoperiodic diapause induction in the parasitoid Nasonia vitripennis in Europe. J. Evol. Biol. 2013, 26, 705–718. [Google Scholar] [CrossRef] [Green Version]
- Gom, T. Geographic variation in critical photoperiod for diapause induction and its temperature dependence in Hyphantria cunea Drury (Lepidoptera: Arctiidae). Oecologia 1997, 111, 160–165. [Google Scholar] [CrossRef]
- van Dyken, J.D.; Wade, M.J. The genetic signature of conditional expression. Genetics 2010, 184, 557–570. [Google Scholar] [CrossRef] [Green Version]
- Nylin, S. Life history perspectives on pest insects: What’s the use? Aust. J. Ecol. 2001, 26, 507–517. [Google Scholar] [CrossRef]
- Falconer, D.S. Selection in different environments: Effects on environmental sensitivity (reaction norm) and on mean performance. Genet. Res. 1990, 56, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Leather, S.R.; Walters, K.F.A.; Bale, J.S. The Ecology of Insect Overwintering; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Savalli, U.M.; Fox, C.W. Sexual selection and the fitness consequences of male body size in the seed beetle, Stator limbatus. Anim. Behav. 1998, 55, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Testa, N.D.; Ghosh, S.M.; Shingleton, A.W. Sex-specific weight loss mediates sexual size dimorphism in Drosophila melanogaster. PLoS ONE 2013, 8, e58936. [Google Scholar] [CrossRef] [PubMed]






| Population | Geographic Position | Rearing Daylength (h) | Developmental Pathway | Sex | N | Female % | Diapause % |
|---|---|---|---|---|---|---|---|
| HB | 44°93′ N 127°17′ E | 15.5 | Non-diapause | ♀ | 87 | 65.9 * | 50.6 |
| ♂ | 45 | ||||||
| Diapause | ♀ | 94 | 69.6 * | ||||
| ♂ | 41 | ||||||
| SY | 42°41′ N 123°29′ E | 15.0 | Non-diapause | ♀ | 85 | 52.5 | 43.9 |
| ♂ | 77 | ||||||
| Diapause | ♀ | 50 | 39.4 * | ||||
| ♂ | 77 | ||||||
| LF | 39°53′ N 116°68′ E | 14.5 | Non-diapause | ♀ | 40 | 46.0 | 74.6 |
| ♂ | 47 | ||||||
| Diapause | ♀ | 127 | 49.6 | ||||
| ♂ | 129 | ||||||
| TA | 36°19′ N 117°09′ E | 14.5 | Non-diapause | ♀ | 74 | 44.3 | 28.9 |
| ♂ | 93 | ||||||
| Diapause | ♀ | 38 | 55.9 | ||||
| ♂ | 30 | ||||||
| HF | 31°81′ N 117°22′ E | 14.0 | Non-diapause | ♀ | 70 | 59.8 * | 59.4 |
| ♂ | 47 | ||||||
| Diapause | ♀ | 103 | 60.2 * | ||||
| ♂ | 68 | ||||||
| YX | 29°04′ N 115°82′ E | 13.5 | Non-diapause | ♀ | 46 | 54.8 | 57.6. |
| ♂ | 38 | ||||||
| Diapause | ♀ | 42 | 36.8 * | ||||
| ♂ | 72 |
| Traits | Fixed Effects | df | F | p |
|---|---|---|---|---|
| Larval time | Population | 5 | 11.051 | <0.001 |
| Sex | 1 | 0.517 | 0.472 | |
| Dvpt. path. | 1 | 1411.443 | <0.001 | |
| Population × Sex | 5 | 3.633 | 0.003 | |
| Population × Dvpt. path. | 5 | 21.820 | <0.001 | |
| Sex × Dvpt. path. | 1 | 3.777 | 0.052 | |
| Population × Sex × Dvpt. path. | 5 | 8.850 | <0.001 | |
| Pupal time | Population | 5 | 1.092 | 0.363 |
| Sex | 1 | 13.882 | <0.001 | |
| Dvpt. path. | 1 | 2.617 | 0.106 | |
| Population × Sex | 5 | 1.197 | 0.308 | |
| Population × Dvpt. path. | 5 | 1.715 | 0.128 | |
| Sex × Dvpt. path. | 1 | 0.000 | 0.987 | |
| Pupal weight | Population | 5 | 5.508 | <0.001 |
| Sex | 1 | 138.757 | <0.001 | |
| Dvpt. path. | 1 | 79.143 | <0.001 | |
| Population × Sex | 5 | 3.761 | 0.002 | |
| Population × Dvpt. path. | 5 | 3.550 | 0.003 | |
| Sex × Dvpt. path. | 1 | 15.726 | <0.001 | |
| Population × Sex × Dvpt. path. | 5 | 3.053 | 0.010 | |
| Growth rate | Population | 5 | 12.202 | <0.001 |
| Sex | 1 | 3.929 | 0.048 | |
| Dvpt. path. | 1 | 1134.056 | <0.001 | |
| Population × Sex | 5 | 1.425 | 0.212 | |
| Population × Dvpt. path. | 5 | 9.663 | <0.001 | |
| Sex × Dvpt. path. | 1 | 1.617 | 0.204 | |
| Adult weight | Population | 5 | 10.024 | <0.001 |
| Sex | 1 | 261.309 | <0.001 | |
| Dvpt. path. | 1 | 82.125 | <0.001 | |
| Population × Sex | 5 | 6.272 | <0.001 | |
| Population × Dvpt. path. | 5 | 8.500 | <0.001 | |
| Sex × Dvpt. path. | 1 | 27.797 | <0.001 | |
| Population × Sex × Dvpt. path. | 5 | 4.650 | <0.001 | |
| Weight loss | Population | 5 | 2.227 | 0.049 |
| Sex | 1 | 114.932 | <0.001 | |
| Dvpt. path. | 1 | 5.892 | 0.015 | |
| Population × Sex | 5 | 1.467 | 0.198 | |
| Population × Dvpt. path. | 5 | 2.771 | 0.017 | |
| Sex × Dvpt. path. | 1 | 5.101 | 0.024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, S.; Huang, L.; He, H.; Tang, J.; Wu, S.; Xue, F. Differentiation of Developmental Pathways Results in Different Life-History Patterns between the High and Low Latitudinal Populations in the Asian Corn Borer. Insects 2022, 13, 1026. https://doi.org/10.3390/insects13111026
Fu S, Huang L, He H, Tang J, Wu S, Xue F. Differentiation of Developmental Pathways Results in Different Life-History Patterns between the High and Low Latitudinal Populations in the Asian Corn Borer. Insects. 2022; 13(11):1026. https://doi.org/10.3390/insects13111026
Chicago/Turabian StyleFu, Shu, Lili Huang, Haimin He, Jianjun Tang, Shaohui Wu, and Fangsen Xue. 2022. "Differentiation of Developmental Pathways Results in Different Life-History Patterns between the High and Low Latitudinal Populations in the Asian Corn Borer" Insects 13, no. 11: 1026. https://doi.org/10.3390/insects13111026