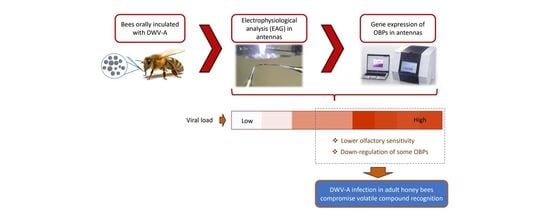
Variant A of the Deformed Wings Virus Alters the Olfactory Sensitivity and the Expression of Odorant Binding Proteins on Antennas of Apis mellifera
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum Preparation
2.2. Bee Inoculation
2.3. Stimulus Preparation
2.4. Antennal Response
2.5. RNA Extraction and cDNA Synthesis
2.6. Real-Time PCR Quantification (qPCR)
2.7. Data Analysis
3. Results
3.1. Electroantennography Responses and Viral Load in Antennas
3.2. Expression of OBP Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguilar, R.; Ashworth, L.; Galetto, L.; Aizen, M.A. Plant reproductive susceptibility to habitat fragmentation: Review and synthesis through a meta-analysis. Ecol. Lett. 2006, 9, 968–980. [Google Scholar] [CrossRef]
- Ricketts, T.H.; Regetz, J.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Bogdanski, A.; Gemmill-Herren, B.; Greenleaf, S.S.; Klein, A.M.; Mayfield, M.M.; et al. Landscape effects on crop pollination services: Are there general patterns? Ecol. Lett. 2008, 11, 499–515. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- vanEngelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony collapse disorder: A descriptive study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Nicholls, E.; Botias, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Semmens, B.X.; Semmens, D.J.; Thogmartin, W.E.; Wiederholt, R.; Lopez-Hoffman, L.; Diffendorfer, J.E.; Pleasants, J.M.; Oberhauser, K.S.; Taylor, O.R. Quasi-extinction risk and population targets for the Eastern, migratory population of monarch butterflies (Danaus plexippus). Sci. Rep. 2016, 6, 23265. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.M.; Steiner, R. Insect pollinator conservation policy innovations at subnational levels: Lessons for lawmakers. Environ. Sci. Policy 2019, 93, 118–128. [Google Scholar] [CrossRef]
- vanEngelsdorp, D.; Caron, D.; Hayes, J.; Underwood, R.; Henson, M.; Rennich, K.; Spleen, A.; Andree, M.; Snyder, R.; Lee, K.; et al. A national survey of managed honey bee 2010–11 winter colony losses in the USA: Results from the Bee Informed Partnership. J. Apic. Res. 2015, 51, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Tlak Gajger, I.; Majoros, A.; Dar, S.A.; Khan, S.; Kalimullah; Haleem Shah, A.; Nasir Khabir, M.; Hussain, R.; Khan, H.U.; et al. Viral impacts on honey bee populations: A review. Saudi J. Biol. Sci. 2021, 28, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Beaurepaire, A.; Piot, N.; Doublet, V.; Antunez, K.; Campbell, E.; Chantawannakul, P.; Chejanovsky, N.; Gajda, A.; Heerman, M.; Panziera, D.; et al. Diversity and global distribution of viruses of the western Honey Bee, Apis. mellifera. Insects 2020, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Tentcheva, D.; Tournaire, M.; Dainat, B.; Cousserans, F.; Colin, M.E.; Bergoin, M. Viral load estimation in asymptomatic honey bee colonies using the quantitative RT-PCR technique. Apidologie 2007, 38, 426–435. [Google Scholar] [CrossRef] [Green Version]
- Mordecai, G.J.; Wilfert, L.; Martin, S.J.; Jones, I.M.; Schroeder, D.C. Diversity in a honey bee pathogen: First report of a third master variant of the deformed Wing Virus quasispecies. ISME J. 2016, 10, 1264–1273. [Google Scholar] [CrossRef] [Green Version]
- Kevill, J.L.; Highfield, A.; Mordecai, G.J.; Martin, S.J.; Schroeder, D.C. ABC Assay: Method development and application to quantify the role of three DWV master variants in overwinter colony losses of European honey bees. Viruses 2017, 9, 314. [Google Scholar] [CrossRef] [Green Version]
- Ongus, J.R.; Peters, D.; Bonmatin, J.M.; Bengsch, E.; Vlak, J.M.; van Oers, M.M. Complete sequence of a picorna-like virus of the genus Iflavirus replicating in the mite Varroa destructor. J. Gen. Virol. 2004, 85, 3747–3755. [Google Scholar] [CrossRef]
- Lanzi, G.; de Miranda, J.R.; Boniotti, M.B.; Cameron, C.E.; Lavazza, A.; Capucci, L.; Camazine, S.M.; Rossi, C. Molecular and biological characterization of deformed wing virus of honeybees (Apis mellifera L.). J. Virol. 2006, 80, 4998–5009. [Google Scholar] [CrossRef] [Green Version]
- Benaets, K.; Van Geystelen, A.; Cardoen, D.; De Smet, L.; de Graaf, D.C.; Schoofs, L.; Larmuseau, M.H.; Brettell, L.E.; Martin, S.J.; Wenseleers, T. Covert deformed wing virus infections have long-term deleterious effects on honeybee foraging and survival. Proc. Biol. Sci. 2017, 284. [Google Scholar] [CrossRef] [PubMed]
- Grozinger, C.M.; Flenniken, M.L. Bee Viruses: Ecology, Pathogenicity, and Impacts. Annu. Rev. Entomol. 2019, 64, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Mueller, U. Virus infection causes specific learning deficits in honeybee foragers. Proc. Biol. Sci. 2007, 274, 1517–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, K.S.; Evans, E.C.; Pizzorno, M.C. Localization of deformed wing virus (DWV) in the brains of the honeybee, Apis mellifera Linnaeus. Virol. J. 2009, 6, 182. [Google Scholar] [CrossRef] [Green Version]
- Mondet, F.; Alaux, C.; Severac, D.; Rohmer, M.; Mercer, A.; Le Conte, Y. Antennae hold a key to Varroa-sensitive hygiene behaviour in honey bees. Sci. Rep. 2015, 5, 10454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Mercer, A.; Mitchell, A.; de Miranda, J.; Ward, V.; Mondet, F.; Bostina, M. Viral infections alter antennal epithelium ultrastructure in honey bees. J. Invertebr. Pathol. 2019, 168, 107252. [Google Scholar] [CrossRef]
- Groot, A.T.; Dekker, T.; Heckel, D.G. The genetic basis of pheromone evolution in moths. Annu. Rev. Entomol. 2016, 61, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liberles, S.D. Aversion and attraction through olfaction. Curr. Biol. 2015, 25, R120–R129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foret, S.; Maleszka, R. Function and evolution of a gene family encoding odorant binding-like proteins in a social insect, the honey bee (Apis mellifera). Genome Res. 2006, 16, 1404–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelosi, P.; Calvello, M.; Ban, L. Diversity of odorant-binding proteins and chemosensory proteins in insects. Chem. Senses 2005, 30 (Suppl. 1), i291–i292. [Google Scholar] [CrossRef] [Green Version]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Zhou, J.-J. Odorant-binding proteins in insects. In Pheromones; Vitamins & Hormones: Harpenden, Hertfordshire, UK, 2010; pp. 241–272. [Google Scholar]
- Deyu, Z.; Leal, W.S. Conformational isomers of insect Odorant-Binding Proteins. Arch. Bioche. Biophys. 2002, 397, 99–105. [Google Scholar] [CrossRef]
- Gusachenko, O.N.; Woodford, L.; Balbirnie-Cumming, K.; Ryabov, E.V.; Evans, D.J. Evidence for and against deformed wing virus spillover from honey bees to bumble bees: A reverse genetic analysis. Sci. Rep. 2020, 10, 16847. [Google Scholar] [CrossRef]
- Skubnik, K.; Novacek, J.; Fuzik, T.; Pridal, A.; Paxton, R.J.; Plevka, P. Structure of deformed wing virus, a major honey bee pathogen. Proc. Natl. Acad. Sci. USA 2017, 114, 3210–3215. [Google Scholar] [CrossRef] [Green Version]
- Vargas, M.; Arismendi, N.; Riveros, G.; Zapata, N.; Bruna, A.; Vidal, M.; Rodríguez, M.; Gerding, M. Viral and intestinal diseases detected in Apis mellifera in Central and Southern Chile. Chil. J. Agric. Res. 2017, 77, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Porrini, M.P.; Garrido, P.M.; Eguaras, M.J. Individual feeding of honey bees: Modification of the Rinderer technique. J. Apic. Res. 2013, 52, 194–195. [Google Scholar] [CrossRef]
- Arismendi, N.; Caro, S.; Castro, M.P.; Vargas, M.; Riveros, G.; Venegas, T. Impact of mixed infections of gut parasites Lotmaria passim and Nosema ceranae on the lifespan and immune-related biomarkers in Apis mellifera. Insects 2020, 11, 420. [Google Scholar] [CrossRef]
- Bobis, O.; Moise, A.R.; Ballesteros, I.; Reyes, E.S.; Duran, S.S.; Sanchez-Sanchez, J.; Cruz-Quintana, S.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Eucalyptus honey: Quality parameters, chemical composition and health-promoting properties. Food Chem. 2020, 325, 126870. [Google Scholar] [CrossRef]
- Eliyahu, A.; Duman, Z.; Sherf, S.; Genin, O.; Cinnamon, Y.; Abu-Abied, M.; Weinstain, R.; Dag, A.; Sadot, E. Vegetative propagation of elite Eucalyptus clones as food source for honeybees (Apis mellifera); adventitious roots versus callus formation. Isr. J. Plant. Sci. 2020, 67, 83–97. [Google Scholar] [CrossRef] [Green Version]
- Terrab, A.; Valdés, B.; Díez, M.J. Study of plants visited by honeybees (Apis mellifera L.) in the Central Rif Region (N. Morocco) using pollen analysis. Grana 2005, 44, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Ciotlaus, I.; Balea, A.; Pojar-Fenesan, M.; Petean, I. Cromathographic profile of volatiles of multifloral and unifloral honey collected by Apis Mellifera from Transilvania, Romania. Rev. Chim. 2020, 71, 91–98. [Google Scholar] [CrossRef]
- Silva, D.; Curkovic, T.; Ceballos, R. Behavioral and antennal responses of Lobesia botrana (Lepidoptera: Tortricidae) to volatiles from the non-host plant Schinus molle L. (Anacardiaceae). Chil. J. Agric. Res. 2019, 79, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Ceballos, R.; Fernández, N.; Zúñiga, S.; Zapata, N. Electrophysiological and behavioral responses of pea weevil Bruchus pisorum L. (Coleóptera: Bruchidae) to volatiles collected from its host Pisum sativum L. Chil. J. Agric. Res. 2015, 75, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 2003–2007. [Google Scholar] [CrossRef] [PubMed]
- Riveros, G.; Arismendi, N.; Zapata, N.; Evans, D.; Pérez, I.; Aldea, P.; Vargas, M. Occurrence, prevalence and viral load of deformed wing virus variants in Apis mellifera colonies in Chile. J. Apic. Res. 2019, 59, 63–68. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, X.; Kadowaki, T. Characterization of the copy number and variants of deformed wing virus (DWV) in the pairs of honey bee pupa and infesting Varroa destructor or Tropilaelaps mercedesae. Front. Microbiol. 2017, 8, 1558. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cox-Foster, D. Impact of an ectoparasite on the immunity and pathology of an invertebrate: Evidence for host immunosuppression and viral amplification. Proc. Natl. Acad. Sci. USA 2005, 102, 7470–7475. [Google Scholar] [CrossRef] [Green Version]
- McMahon, D.P.; Natsopoulou, M.E.; Doublet, V.; Furst, M.; Weging, S.; Brown, M.J.; Gogol-Doring, A.; Paxton, R.J. Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proc. Biol. Sci. 2016, 283. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.; Kefi, S.; Tabben, O.; Ayed, A.; Jallouli, S.; Feres, N.; Hammami, M.; Khammassi, S.; Hrigua, I.; Nefisi, S.; et al. Variation in chemical composition of Eucalyptus globulus essential oil under phenological stages and evidence synergism with antimicrobial standards. Ind. Crop. Prod. 2018, 124, 115–125. [Google Scholar] [CrossRef]
- Jerbi, A.; Derbali, A.; Elfeki, A.; Kammoun, M. Essential oil composition and biological activities of Eucalyptus globulus leaves extracts from Tunisia. J. Essent Oil. Bear Plants 2017, 20, 438–448. [Google Scholar] [CrossRef]
- Moghaddam, M.; Pourbaige, M.; Tabar, H.K.; Farhadi, N.; Hosseini, S.M.A. Composition and antifungal activity of Peppermint (Mentha piperita) essential oil from Iran. J. Essent. Oil Bear. Plants 2013, 16, 506–512. [Google Scholar] [CrossRef]
- Yong, W.T.L.; Ades, P.K.; Goodger, J.Q.D.; Bossinger, G.; Runa, F.A.; Sandhu, K.S.; Tibbits, J.F.G. Using essential oil composition to discriminate between myrtle rust phenotypes in Eucalyptus globulus and Eucalyptus obliqua. Ind. Crop. Prod. 2019, 140, 111595. [Google Scholar] [CrossRef]
- Wiese, N.; Fischer, J.; Heidler, J.; Lewkowski, O.; Degenhardt, J.; Erler, S. The terpenes of leaves, pollen, and nectar of thyme (Thymus vulgaris) inhibit growth of bee disease-associated microbes. Sci. Rep. 2018, 8, 14634. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.W.; Park, K.W.; Oh, H.-W.; Kwon, H.W. Structural and functional differences in the antennal olfactory system of worker honey bees of Apis mellifera and Apis cerana. J. Asia-Pac. Entomol. 2014, 17, 639–646. [Google Scholar] [CrossRef]
- Larson, N.R.; O’Neal, S.T.; Bernier, U.R.; Bloomquist, J.R.; Anderson, T.D. Terpenoid-induced feeding deterrence and antennal response of honey bees. Insects 2020, 11, 83. [Google Scholar] [CrossRef] [Green Version]
- Holt, H.L.; Villar, G.; Cheng, W.; Song, J.; Grozinger, C.M. Molecular, physiological and behavioral responses of honey bee (Apis mellifera) drones to infection with microsporidian parasites. J. Invertebr. Pathol. 2018, 155, 14–24. [Google Scholar] [CrossRef]
- Vieira, F.G.; Rozas, J. Comparative genomics of the odorant-binding and chemosensory protein gene families across the Arthropoda: Origin and evolutionary history of the chemosensory system. Genome Biol. Evol. 2011, 3, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Iovinella, I.; Zhu, J.; Wang, G.; Dani, F.R. Beyond chemoreception: Diverse tasks of soluble olfactory proteins in insects. Biol. Rev. Camb. Philos. Soc. 2018, 93, 184–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelosi, P.; Iovinella, I.; Felicioli, A.; Dani, F.R. Soluble proteins of chemical communication: An overview across arthropods. Front. Physiol. 2014, 5, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, H.; Tuccori, E.; He, X.; Gazzano, A.; Field, L.; Zhou, J.J.; Pelosi, P. Discrimination of alarm pheromone (E)-beta-farnesene by aphid odorant-binding proteins. Insect Biochem. Mol. Biol. 2009, 39, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.; Guidolin, A.; Syed, Z.; Cornel, A.J.; Leal, W.S. Knockdown of a mosquito odorant-binding protein involved in the sensitive detection of oviposition attractants. J. Chem. Ecol. 2010, 36, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Briand, L.; Nespoulous, C.; Huet, J.C.; Takahashi, M.; Pernollet, J.C. Ligand binding and physico-chemical properties of ASP2, a recombinant odorant-binding protein from honeybee (Apis mellifera L.). Eur. J. Biochem. 2001, 268, 752–760. [Google Scholar] [CrossRef]
- Zhao, H.; Zeng, X.; Liang, Q.; Zhang, X.; Huang, W.; Chen, H.; Luo, Y. Study of the obp5 gene in Apis mellifera ligustica and Apis cerana cerana. Genet. Mol. Res. 2015, 14, 6482–6494. [Google Scholar] [CrossRef]
- Zhao, H.; Luo, Y.; Lee, J.; Zhang, X.; Liang, Q.; Zeng, X. The Odorant-binding protein gene obp11 shows different spatiotemporal roles in the olfactory system of Apis mellifera ligustica and Apis cerana cerana. Sociobiology 2013, 60, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Nie, H.; Xu, S.; Xie, C.; Geng, H.; Zhao, Y.; Li, J.; Huang, W.F.; Lin, Y.; Li, Z.; Su, S. Comparative transcriptome analysis of Apis mellifera antennae of workers performing different tasks. Mol. Genet. Genomics 2018, 293, 237–248. [Google Scholar] [CrossRef]
- Johnson, B.R. Division of labor in honeybees: Form, function, and proximate mechanisms. Behav. Ecol. Sociobiol. 2010, 64, 305–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortolotti, L.; Costa, C. Chemical communication in the honey bee society. In Neurobiology of Chemical Communication; Mucignat-Caretta, C., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Traniello, I.M.; Bukhari, S.A.; Kevill, J.; Ahmed, A.C.; Hamilton, A.R.; Naeger, N.L.; Schroeder, D.C.; Robinson, G.E. Meta-analysis of honey bee neurogenomic response links deformed wing virus type A to precocious behavioral maturation. Sci. Rep. 2020, 10, 3101. [Google Scholar] [CrossRef] [Green Version]
- Wells, T.; Wolf, S.; Nicholls, E.; Groll, H.; Lim, K.S.; Clark, S.J.; Swain, J.; Osborne, J.L.; Haughton, A.J. Flight performance of actively foraging honey bees is reduced by a common pathogen. Environ. Microbiol. Rep. 2016, 8, 728–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kevill, J.L.; de Souza, F.S.; Sharples, C.; Oliver, R.; Schroeder, D.C.; Martin, S.J. DWV-A lethal to honey bees (Apis mellifera): A colony level survey of DWV variants (A, B, and C) in England, Wales, and 32 States across the US. Viruses 2019, 11, 426. [Google Scholar] [CrossRef] [Green Version]
- Ryabov, E.V.; Childers, A.K.; Lopez, D.; Grubbs, K.; Posada-Florez, F.; Weaver, D.; Girten, W.; van Engelsdorp, D.; Chen, Y.; Evans, J.D. Dynamic evolution in the key honey bee pathogen deformed wing virus: Novel insights into virulence and competition using reverse genetics. PLoS Biol. 2019, 17, e3000502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryabov, E.V.; Wood, G.R.; Fannon, J.M.; Moore, J.D.; Bull, J.C.; Chandler, D.; Mead, A.; Burroughs, N.; Evans, D.J. A virulent strain of deformed wing virus (DWV) of honey bees (Apis mellifera) prevails after Varroa destructor-mediated, or in vitro, transmission. PLoS Pathog. 2014, 10, e1004230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesovnik, T.; Zorc, M.; Ristanic, M.; Glavinic, U.; Stevanovic, J.; Narat, M.; Stanimirovic, Z. Exposure of honey bee larvae to thiamethoxam and its interaction with Nosema ceranae infection in adult honey bees. Environ. Pollut. 2020, 256, 113443. [Google Scholar] [CrossRef]
- Martin, S.J.; Highfield, A.C.; Brettell, L.; Villalobos, E.M.; Budge, G.E.; Powell, M.; Nikaido, S.; Schroeder, D.C. Global honey bee viral landscape altered by a parasitic mite. Science 2012, 336, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Brettell, L.E. Deformed wing virus in boneybees and other insects. Annu. Rev. Virol. 2019, 6, 49–69. [Google Scholar] [CrossRef] [Green Version]
- Wilfert, L.; Long, G.; Leggett, H.C.; Schmid-Hempel, P.; Butlin, R.; Martin, S.J.M.; Boots, M. Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science 2016, 351, 594–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tritschler, M.; Vollmann, J.J.; Yanez, O.; Chejanovsky, N.; Crailsheim, K.; Neumann, P. Protein nutrition governs within-host race of honey bee pathogens. Sci. Rep. 2017, 7, 14988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barroso-Arevalo, S.; Fernandez-Carrion, E.; Goyache, J.; Molero, F.; Puerta, F.; Sanchez-Vizcaino, J.M. High load of deformed wing virus and Varroa destructor infestation are related to weakness of honey bee colonies in southern Spain. Front. Microbiol. 2019, 10, 1331. [Google Scholar] [CrossRef] [PubMed]





| Primer | Sequence (5′–3′) | References | |
|---|---|---|---|
| AmelOBP1 | F | ACCTGGTAAACGAACCGTCCA | [23] |
| R | TCAACACAGCCTGTTCTCGA | ||
| AmelOBP2 | F | TCTGACCGTTGTACGTGGCA | [23] |
| R | TGGCATTCTCGATGCACTCA | ||
| AmelOBP4 | F | TGCGCTGGTTCACGCAGACA | [23] |
| R | ATGCATTCGTCTTCGTCTGCA | ||
| AmelOBP5 | F | ATGCGGAAATCGTGCTTGCA | [23] |
| R | TGCCATTACTCACGGGAAGA | ||
| AmelOBP8 | F | GTTGCGTGGCAATTTGGCAAATG | [23] |
| R | TACGTTGTTTCGCCCTTCCAGT | ||
| AmelOBP11 | F | TGAGGATGTCGAAGCTACGGAA | [23] |
| R | CACGGAGCAATAAACGCTATGG | ||
| AmelOBP12 | F | TGCGTGGATCGATCAAACATGA | [23] |
| R | ACGTTAACGCGATCTTATGGA | ||
| AmelOBP15 | F | TTGCATGGCAAAAACTGGCA | [23] |
| R | TCTCTGGATACGTGTTCGTTGA | ||
| β-Actin | F | ATGCCAACACTGTCCTTTCTGG | [43] |
| R | GACCCACCAATCCATACGGA | ||
| DWV-A | F | TATCTTCATTAAAGCCACCTGGAA | [44] |
| R | TTTCCTCATTAACTGTGTCGTTGAT |
| First Variable | Second Variable | rs | t-Value | p-Value |
|---|---|---|---|---|
| DWV-A load | Relative expression | |||
| OBP2 | 0.45 | 2.03 | 0.059 | |
| OBP5 | 0.25 | 1.04 | 0.316 | |
| OBP12 | −0.69 | 3.86 | 0.001 | |
| OBP11 | 0.21 | 0.86 | 0.404 | |
| EAG values | ||||
| Mp 0.1 mg/mL | −0.76 | 4.72 | <0.001 | |
| Mp 1.0 mg/mL | −0.70 | 3.88 | 0.001 | |
| Mp 10.0 mg/mL | −0.83 | 5.88 | <0.001 | |
| Eg 0.1 mg/mL | 0.05 | 0.22 | 0.829 | |
| Eg 1.0 mg/mL | −0.28 | 1.17 | 0.261 | |
| Eg 10.0 mg/mL | −0.11 | 0.44 | 0.663 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, D.; Ceballos, R.; Arismendi, N.; Dalmon, A.; Vargas, M. Variant A of the Deformed Wings Virus Alters the Olfactory Sensitivity and the Expression of Odorant Binding Proteins on Antennas of Apis mellifera. Insects 2021, 12, 895. https://doi.org/10.3390/insects12100895
Silva D, Ceballos R, Arismendi N, Dalmon A, Vargas M. Variant A of the Deformed Wings Virus Alters the Olfactory Sensitivity and the Expression of Odorant Binding Proteins on Antennas of Apis mellifera. Insects. 2021; 12(10):895. https://doi.org/10.3390/insects12100895
Chicago/Turabian StyleSilva, Diego, Ricardo Ceballos, Nolberto Arismendi, Anne Dalmon, and Marisol Vargas. 2021. "Variant A of the Deformed Wings Virus Alters the Olfactory Sensitivity and the Expression of Odorant Binding Proteins on Antennas of Apis mellifera" Insects 12, no. 10: 895. https://doi.org/10.3390/insects12100895