Role of Ovarian Proteins Secreted by Toxoneuron nigriceps (Viereck) (Hymenoptera, Braconidae) in the Early Suppression of Host Immune Response
Abstract
:Simple Summary
Abstract
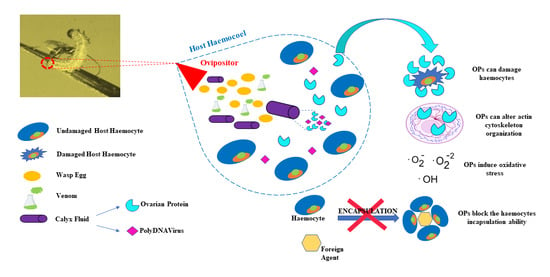
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Calyx Fluid Collection and Ovarian Protein Purification
2.3. Collection of Haemocytes from Larvae of H. virescens
2.4. Cells Viability
2.5. Light Microscopy Haemocyte Observations
2.6. Chromatographic Sphere Staining
2.7. Encapsulation Measurement
2.8. Injections of OPs and Chromatographic Spheres in H. virescens Larvae and Evaluation of In Vivo Encapsulation Degree
2.9. Statistical Analysis of Data
3. Results
3.1. Cell Viability of the Haemocytes after Treatment with Ovarian Proteins
3.2. Haemocyte Staining
3.2.1. May Grunwald–Giemsa Staining
3.2.2. H2DCFDA Staining
3.2.3. TRITC-Conjugated Phalloidin Staining
3.3. In Vivo Encapsulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Quicke, D.L.J. Parasitic Wasps; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Heraty, J. Parasitoid Biodiversity and Insect Pest Management. In Insect Biodiversity: Science and Society, 2nd ed.; Foottit, R.G., Adler, P.H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 603–625. [Google Scholar]
- Godfray, H.C.J. Parasitoids. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–13. [Google Scholar]
- Pennacchio, F.; Strand, M.R. Evolution of developmental strategies in parasitic Hymenoptera. Annu. Rev. Entomol. 2006, 51, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, O.; Theopold, U.; Strand, M.R. Innate immunity and evasion by insect parasitoids. BioEssays 2001, 23, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Bangham, J.; Jiggins, F.; Lemaitre, B. Insect immunity: The post-genomic era. Immunity 2006, 25, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheehan, G.; Garvey, A.; Croke, M.; Kavanagh, K. Innate humoral immune defences in mammals and insects: The same, with differences? Virulence 2018, 9, 1625–1639. [Google Scholar] [CrossRef] [Green Version]
- Brivio, M.F.; Mastore, M. When Appearance Misleads: The Role of the Entomopathogen Surface in the Relationship with Its Host. Insects 2020, 11, 387. [Google Scholar] [CrossRef]
- Welchman, D.P.; Aksoy, S.; Jiggins, F.; Lemaitre, B. Insect immunity: From pattern recognition to symbiont-mediated host defense. Cell Host Microbe 2009, 6, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhang, Y.; Zhang, R.; Zhang, J. The diversity of pattern recognition receptors (PRRs) involved with insect defense against pathogens. Curr. Opin. Insect Sci. 2019, 33, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, G.; Farrell, G.; Kavanagh, K. Immune priming: The secret weapon of the insect world. Virulence 2020, 11, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Nakhleh, J.; Moussawi, L.E.; Osta, M.A. Chapter Three—The Melanization Response in Insect Immunity. Adv. Insect Physiol. 2017, 52, 83–109. [Google Scholar]
- Asgari, S. Venoms from Endoparasitoids. In Parasitoid Viruses; Beckage, N.E., Drezen, J.M., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 217–231. [Google Scholar]
- Strand, M.R. Teratocytes and their functions in parasitoids. Curr. Opin. Insect Sci. 2014, 6, 68–73. [Google Scholar] [CrossRef]
- Laurino, S.; Grossi, G.; Pucci, P.; Flagiello, A.; Bufo, S.A.; Bianco, G.; Salvia, R.; Vinson, S.B.; Vogel, H.; Falabella, P. Identification of major Toxoneuron nigriceps venom proteins using an integrated transcriptomic/proteomic approach. Insect Biochem. Mol. Biol. 2016, 76, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Leobold, M.; Bézier, A.; Pichon, A.; Herniou, E.A.; Volkoff, A.N.; Drezen, J.M. The domestication of a large DNA virus by the wasp Venturia canescens involves targeted genome reduction through pseudogenization. Genome Biol. Evol. 2018, 10, 1745–1764. [Google Scholar] [CrossRef] [PubMed]
- Salvia, R.; Grimaldi, A.; Girardello, R.; Scieuzo, C.; Scala, A.; Bufo, S.A.; Vogel, H.; Falabella, P. Aphidius ervi Teratocytes Release Enolase and Fatty Acid Binding Protein Through Exosomal Vesicles. Front. Physiol. 2019, 10, 715. [Google Scholar] [CrossRef]
- Fath-Goodin, A.; Webb, B.A. Polydnaviruses: General Features. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 256–261. [Google Scholar]
- Strand, M.R.; Burke, G.R. Polydnaviruses: From discovery to current insights. Virology 2015, 479–480, 393–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkoff, A.N.; Huguet, E. Polydnaviruses (Polydnaviridae, Bracovirus, and Ichnovirus). In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Lovallo, N.C.; McPherson, B.A.; Cox-Foster, D.L. Effects of the polydnavirus of Cotesia congregata on the immune system and development of non-habitual hosts of the parasitoid. J. Insect Physiol. 2002, 48, 517–526. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, C.; Qin, J.; Wang, C. Polydnavirus of Campoletis chlorideae: Characterization and temporal effect on host Helicoverpa armigera cellular immune response. Arch. Insect Biochem. Physiol. 2003, 52, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Barat-Houari, M.; Hilliou, F.; Jousset, F.X.; Sofer, L.; Deleury, E.; Rocher, J.; Ravallec, M.; Galibert, L.; Delobel, P.; Feyereisen, R.; et al. Gene expression profiling of Spodoptera frugiperda hemocytes and fat body using cDNA microarray reveals polydnavirus-associated variations in lepidopteran host genes transcript levels. BMC Genom. 2006, 7, 160. [Google Scholar] [CrossRef] [Green Version]
- Falabella, P.; Varricchio, P.; Provost, B.; Espagne, E.; Ferrarese, R.; Grimaldi, A.; de Eguileor, M.; Fimiani, G.; Ursini, M.V.; Malva, C.; et al. Characterization of the IkappaB-like gene family in polydnaviruses associated with wasps belonging to different Braconid subfamilies. J. Gen. Virol. 2007, 88, 92–104. [Google Scholar] [CrossRef]
- Gueguen, G.; Kalamarz, M.E.; Ramroop, J.; Uribe, J.; Govind, S. Polydnaviral Ankyrin Proteins Aid Parasitic Wasp Survival by Coordinate and Selective Inhibition of Hematopoietic and Immune NF-kappa B Signaling in Insect Hosts. PLoS Pathog. 2013, 9, e1003580. [Google Scholar] [CrossRef] [Green Version]
- Salvia, R.; Grossi, G.; Amoresano, A.; Scieuzo, C.; Nardiello, M.; Giangrande, C.; Laurenzana, I.; Ruggieri, V.; Bufo, S.A.; Vinson, S.B.; et al. The multifunctional polydnavirus TnBVANK1 protein: Impact on host apoptotic pathway. Sci. Rep. 2017, 7, 11775. [Google Scholar] [CrossRef]
- Ye, X.Q.; Shi, M.; Huang, J.H.; Chen, X.X. Parasitoid polydnaviruses and immune interaction with secondary hosts. Dev. Comp. Immunol. 2018, 83, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Matsumoto, H.; Hayakawa, Y. Analysis in the course of polydnavirus replication in ovarian calyx cells of the parasitoid wasp, Cotesia kariyai (Hymenoptera: Braconidae). Appl. Entomol. Zool. 2002, 37, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Park, B.; Yonggyun, K. Polydnavirus replication at ovarian calyx in Cotesia plutellae and endocrine impact. Korean J. Appl. Entomol. 2010, 43, 225–231. [Google Scholar]
- Lewis, W.J.; Vinson, S.B. Egg and larval development of Cardiochiles nigriceps. Ann. Entomol. Soc. Am. 1968, 61, 561–565. [Google Scholar] [CrossRef]
- Pennacchio, F.; Vinson, S.B.; Tremblay, E. Growth and development of Cardiochiles nigriceps Viereck (Hymenoptera, Braconidae) larvae and their synchronization with some changes of the hemolymph composition of their host, Heliothis virescens (F.) (Lepidoptera, Noctuidae). Arch. Insect Biochem. Physiol. 1993, 24, 65–77. [Google Scholar] [CrossRef]
- Pennacchio, F.; Vinson, S.B.; Tremblay, E.; Tanaka, T. Biochemical and developmental alterations of Heliothis virescens (F.) (Lepidoptera, Noctuidae) larvae induced by the endophagous parasitoid Cardiochiles nigriceps (Hymenoptera, Braconidae). Arch. Insect Biochem. Physiol. 1994, 26, 211–233. [Google Scholar] [CrossRef]
- Pennacchio, F.; Sordetti, R.; Falabella, P.; Vinson, S.B. Biochemical and ultrastructural alterations in prothoracic glands of Heliothis virescens (F.) (Lepidoptera: Noctuidae) last instar larvae parasitized by Cardiochiles nigriceps Viereck (Hymenoptera: Braconidae). Insect Biochem. Molec. Biol. 1997, 27, 439–450. [Google Scholar] [CrossRef]
- Pennacchio, F.; Falabella, P.; Sordetti, R.; Varricchio, P.; Malva, C.; Vinson, S.B. Prothoracic gland inactivation in Heliothis virescens (F.) (Lepidoptera: Noctuidae) larvae parasitized by Cardiochiles nigriceps Viereck (Hymenoptera: Braconidae). J. Insect Physiol. 1998, 44, 845–857. [Google Scholar] [CrossRef]
- Tanaka, T.; Vinson, S.B. Depression of prothoracic gland activity of Heliothis virescens by venom and calix fluid from the parasitoid, Cardiochiles nigriceps. J. Insect Physiol. 1991, 37, 139–144. [Google Scholar] [CrossRef]
- Pennacchio, F.; Vinson, S.B.; Tremblay, E.; Ostuni, A. Alteration of ecdysone metabolism in Heliothis virescens (F.) (Lepidoptera: Noctuidae) larvae induced by Cardiochiles nigriceps Viereck (Hymenoptera: Braconidae) teratocytes. Insect Biochem. Mol. Biol. 1994, 24, 383–394. [Google Scholar] [CrossRef]
- Falabella, P.; Caccialupi, P.; Varricchio, P.; Malva, C.; Pennacchio, F. Protein Tyrosine Phosphatases of Toxoneuron nigriceps bracovirus as potential disrupters of host prothoracic glands. Arch. Insect Biochem. Physiol. 2006, 61, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Salvia, R.; Nardiello, M.; Scieuzo, C.; Scala, A.; Bufo, S.A.; Rao, A.; Vogel, H.; Falabella, P. Novel Factors of Viral Origin Inhibit TOR Pathway Gene Expression. Front Physiol. 2018, 9, 1678. [Google Scholar] [CrossRef] [Green Version]
- Ferrarese, R.; Brivio, M.; Congiu, T.; Falabella, P.; Grimaldi, A.; Mastore, M.; Perletti, G.; Pennacchio, F.; Sciacca, L.; Tettamanti, G.; et al. Early suppression of immune response in Heliothis virescens larvae by the endophagous parasitoid Toxoneuron nigriceps. Invert. Surv. J. 2005, 2, 60–68. [Google Scholar]
- Pennacchio, F.; Vinson, S.B.; Tremblay, E. Morphology and ultrastructure of the serosal cells (teratocytes) in Cardiochiles nigriceps Viereck (Hymenoptera: Braconidae) embryos. Int. J. Insect Morphol. Embryol. 1994, 23, 93–104. [Google Scholar] [CrossRef]
- Pennacchio, F.; Vinson, S.B.; Tremblay, E. Host regulation effects of Heliothis virescens (F.) larvae induced by teratocytes of Cardiochiles nigriceps Viereck (Lepidoptera, Noctuidae—Hymenoptera, Braconidae). Arch. Insect Biochem. Physiol. 1992, 19, 177–192. [Google Scholar] [CrossRef]
- Pennacchio, F.; Vinson, S.B.; Malva, C. Regulation of host endocrine system by the endophagous braconid Cardiochiles nigriceps and its polydnavirus. In Endocrine Interactions of Insect Parasites and Pathogens; Edwards, J.P., Weaver, R.J., Eds.; BIOS Scientific Publishers: Oxford, UK, 2001; pp. 123–132. [Google Scholar]
- Grimaldi, A.; Caccia, S.; Congiu, T.; Ferrarese, R.; Tettamanti, G.; Rivas-Pena, M.; Perletti, G.; Valvassori, R.; Giordana, B.; Falabella, P.; et al. Structure and function of the extraembryonic membrane persisting around the larvae of the parasitoid Toxoneuron nigriceps. J. Insect Physiol. 2006, 52, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Falabella, P. The mechanism utilized by Toxoneuron nigriceps in inhibiting the host immune system. Invert. Surviv. J. 2018, 15, 240–255. [Google Scholar]
- Varricchio, P.; Falabella, P.; Sordetti, R.; Graziani, F.; Malva, C.; Pennacchio, F. Cardiochiles nigriceps polydnavirus: Molecular characterization and gene expression in parasitized Heliothis virescens larvae. Insect Biochem. Mol. Biol. 1999, 29, 1087–1096. [Google Scholar] [CrossRef]
- Lapointe, R.; Wilson, R.; Vilaplana, L.; O’reilly, D.R.; Falabella, P.; Douris, V.; Bernier-Cardou, M.; Pennacchio, F.; Iatrou, K.; Malva, C.; et al. Expression of a Toxoneuron nigriceps polydnavirus-encoded protein causes apoptosis-like programmed cell death in lepidopteran insect cells. J. Gen. Virol. 2005, 86, 963–971. [Google Scholar] [CrossRef]
- Davies, D.H.; Vinson, S.B. Passive evasion by eggs of braconid parasitoid Cardiochiles nigriceps of encapsulation in vitro by hemocytes of host Heliothis virescens. Possible role for fibrous layer in immunity. J. Insect Physiol. 1986, 32, 1003–1010. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, X.X.; Fu, W.J. Passive evasion of encapsulation in Macrocentrus cingulum Brischke (Hymenoptera: Braconidae), a polyembryonic parasitoid of Ostrinia furnacalis Guenee (Lepidoptera: Pyralidae). J. Insect Physiol. 2003, 49, 367–375. [Google Scholar] [CrossRef]
- Teng, Z.; Wu, H.; Ye, X.; Xiong, S.; Xu, G.; Wang, F.; Fang, Q.; Ye, G. An Ovarian Protein Involved in Passive Avoidance of an Endoparasitoid to Evade Its Host Immune Response. J. Proteome Res. 2019, 18, 2695–2705. [Google Scholar] [CrossRef] [PubMed]
- Vinson, S.B.; Guillot, F.S.; Hays, D.B. Rearing of Cardiochiles nigriceps in the laboratory, with Heliothis virescens as hosts. Ann. Entomol. Soc. Am. 1973, 66, 1170–1172. [Google Scholar] [CrossRef]
- Vanderzant, E.S.; Richardson, C.D.; Fort, S.W., Jr. Rearing of the bollworm on artificial diet. J. Econ. Entomol. 1962, 55, 140. [Google Scholar] [CrossRef]
- Beckage, N.E.; Gelman, D.B. Wasp parasitoid disruption of host development: Implication for new biologically based strategies for insect control. Annu. Rev. Entomol. 2004, 49, 299–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, O. Insect Immune Recognition and Suppression. In Insect Immunology; Beckage, N.E., Ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 271–294. [Google Scholar]
- Mrinalini; Werren, J.H. Parasitoid Wasps and Their Venoms. In Evolution of Venomous Animals and Their Toxins; Gopalakrishnakone, P., Malhotra, A., Eds.; Toxinology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–26. [Google Scholar]
- Moreau, S.J.; Asgari, S. Venom Proteins from Parasitoid Wasps and Their Biological Functions. Toxins 2015, 7, 2385–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Z.W.; Xiong, S.J.; Xu, G.; Gan, S.Y.; Chen, X.; Stanley, D.; Yan, Z.C.; Ye, G.Y.; Fang, Q. Protein Discovery: Combined Transcriptomic and Proteomic Analyses of Venom from the Endoparasitoid Cotesia chilonis (Hymenoptera: Braconidae). Toxins 2017, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Volkoff, A.N.; Cusson, M. The Unconventional Viruses of Ichneumonid Parasitoid Wasps. Viruses 2020, 12, 1170. [Google Scholar] [CrossRef]
- Strand, M.R. Chapter 12—Polydnavirus Gene Products that Interact with the Host Immune System. In Parasitoid Viruses; Beckage, N.E., Drezen, J.M., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 149–161. [Google Scholar]
- Bitra, K.; Zhang, S.; Strand, M.R. Transcriptomic profiling of Microplitis demolitor bracovirus reveals host, tissue and stage-specific patterns of activity. J. Gen. Virol. 2011, 92, 2060–2071. [Google Scholar] [CrossRef]
- Johner, A.; Lanzrein, B. Characterization of two genes of the polydnavirus of Chelonus inanitus and their stage-specific expression in the host Spodoptera littoralis. J. Gen. Virol. 2002, 83, 1075–1085. [Google Scholar] [CrossRef]
- Falabella, P.; Varricchio, P.; Gigliotti, S.; Tranfaglia, A.; Pennacchio, F.; Malva, C. Toxoneuron nigriceps polydnavirus encodes a putative aspartyl protease highly expressed in parasitised host larvae. Insect Mol. Biol. 2003, 12, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provost, B.; Varricchio, P.; Arana, E.; Espagne, E.; Falabella, P.; Huguet, E.; La Scaleia, R.; Cattolico, L.; Poirié, M.; Malva, C.; et al. Bracovirus contain a large multigene family coding for a protein tyrosine phosphatase. J. Virol. 2004, 78, 13090–13103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupas, S.; Wanjiru Gitau, C.; Branca, A.; Le Rü, B.P.; Silvain, J.F. Evolution of a Polydnavirus Gene in Relation to Parasitoid–Host Species Immune Resistance. J. Hered. 2008, 99, 491–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desjardins, C.A.; Gundersen-Rindal, D.E.; Hostetler, J.B.; Tallon, L.J.; Fadrosh, D.W.; Fuester, R.W.; Pedroni, M.J.; Haas, B.J.; Schatz, M.C.; Jones, K.M.; et al. Comparative genomics of mutualistic viruses of Glyptapanteles parasitic wasps. Genome Biol. 2008, 9, R183. [Google Scholar] [CrossRef] [Green Version]
- Legeai, F.; Santos, B.; Robin, S.; Bretaudeau, A.; Dikow, R.; Lemaitre, C.; Jouan, V.; Ravallec, M.; Drezen, J.M.; Tagu, D.; et al. Conserved and specific genomic features of endogenous polydnaviruses revealed by whole genome sequencing of two ichneumonid wasps. bioRxiv 2019, in press. [Google Scholar] [CrossRef]
- Carton, Y.; Poirié, M.; Nappi, A. Insect immune resistance to parasitoids. Insect Science 2008, 15, 67–87. [Google Scholar] [CrossRef]
- Webb, B.A.; Luckhart, S. Evidence for an early immunosuppressive role for related Campoletis sonorensis venom and ovarian proteins in Heliothis virescens. Arch. Insect Biochem. Physiol. 1994, 26, 147–163. [Google Scholar] [CrossRef]
- Vincent, B.; Kaeslin, M.; Roth, T.; Heller, M.; Poulain, J.; Cousserans, F.; Schaller, J.; Poirié, M.; Lanzrein, B.; Drezen, J.; et al. The venom composition of the parasitic wasp Chelonus inanitus resolved by combined expressed sequence tags analysis and proteomic approach. BMC Genom. 2010, 11, 693. [Google Scholar] [CrossRef] [Green Version]
- Goecks, J.; Mortimer, N.T.; Mobley, J.A.; Bowersock, G.J.; Taylor, J.; Schlenke, T.A. Integrative approach reveals composition of endoparasitoid wasp venoms. PLoS ONE 2013, 8, e64125. [Google Scholar] [CrossRef]
- Yan, Z.C.; Fang, Q.; Wang, L.; Liu, J.; Zhu, Y.; Wang, F.; Li, F.; Werren, J.H.; Ye, G. Insights into the venom composition and evolution of an endoparasitoid wasp by combining proteomic and transcriptomic analyses. Sci. Rep. 2016, 6, 19604. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Lin, Z.; Fang, Q.; Wang, J.; Yan, Z.; Zou, Z.; Song, Q.; Ye, G. Identification and characterization of serine protease inhibitors in a parasitic wasp, Pteromalus puparum. Sci. Rep. 2017, 7, 15755. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.; Schmidt, O. Passive protection of eggs from parasitoid, Cotesia rubecula, in the host Pieris rapae. J. Insect Physiol. 1994, 40, 789–795. [Google Scholar] [CrossRef]
- Beckage, N.E. Parasitoids and polydnaviruses. Bioscience 1998, 48, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Han, L.B.; Yin, L.H.; Huang, L.Q.; Wang, C.Z. Differential immunosuppression by Campoletis chlorideae eggs and ichnovirus in larvae of Helicoverpa armigera and Spodoptera exigua. J. Invertebr. Pathol. 2015, 130, 88–96. [Google Scholar] [CrossRef]
- Yin, C.; Li, M.; Hu, J.; Lang, K.; Chen, Q.; Liu, J.; Guo, D.; He, K.; Dong, Y.; Luo, J.; et al. The genomic features of parasitism, Polyembryony and immune evasion in the endoparasitic wasp Macrocentrus cingulum. BMC Genom. 2018, 19, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luckhart, S.; Webb, B.A. Interaction of a wasp ovarian protein and polydnavirus in host immune suppression. Dev. Comp. Immunol. 1996, 20, 1–21. [Google Scholar] [CrossRef]
- Bras, M.; Queenan, B.; Susin, S.A. Programmed Cell Death via Mitochondria: Different Modes of Dying. Biochemistry 2005, 70, 231–239. [Google Scholar] [CrossRef]
- Gourlay, C.W.; Ayscough, K.R. A Role for Actin in Aging and Apoptosis. Biochem. Soc. Trans. 2005, 33, 1260–1264. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Li, T.S.; Marbán, E. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells 2010, 28, 1178–1185. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhou, Z.; Min, W. Mitochondria, Oxidative Stress and Innate Immunity. Front. Physiol. 2018, 9, 1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefani, M.; Dobson, C.M. Protein aggregation and aggregate toxicity: New insights into protein folding, misfolding diseases and biological evolution. J. Mol. Med. 2003, 81, 678–699. [Google Scholar] [CrossRef] [PubMed]
- Milkovic, L.; Cipak, G.A.; Cindric, M.; Mouthuy, P.A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Lu, J.F.; Feng, C.J.; Ke, X.; Fu, W.J. Role of venom and ovarian proteins in immune suppression of Ostrinia furnacalis (Lepidoptera: Pyralidae) larvae parasitized by Macrocentrus cingulum (Hymenoptera: Braconidae), a polyembryonic parasitoid. Insect Sci. 2007, 14, 93–100. [Google Scholar] [CrossRef]
- Choi, J.Y.; Whitten, M.M.; Cho, M.Y.; Lee, K.Y.; Kim, M.S.; Ratcliffe, N.A.; Lee, B.L. Calreticulin enriched as an early-stage encapsulation protein in wax moth Galleria mellonella larvae. Dev. Comp. Immunol. 2002, 26, 335–343. [Google Scholar] [CrossRef]
- Asgari, S.; Schmidt, O. Is cell surface calreticulin involved in phagocytosis by insect hemocytes? J. Insect. Physiol. 2003, 49, 545–550. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Fang, Q.; Wang, L.; Hu, C.; Ye, G.Y. Proteomic analysis of the venom from the endoparasitoid wasp Pteromalus puparum (Hymenoptera: Pteromalidae). Arch. Insect Biochem. Physiol. 2010, 75, 28–44. [Google Scholar] [CrossRef]
- Wang, L.; Fang, Q.; Qian, C.; Wang, F.; Yu, X.Q.; Ye, G. Inhibition of host cell encapsulation through inhibiting immune gene expression by the parasitic wasp venom calreticulin. Insect Biochem. Mol. Biol. 2013, 43, 936–946. [Google Scholar] [CrossRef]
- Teng, Z.W.; Xu, G.; Gan, S.Y.; Chen, X.; Fang, Q.; Ye, G.Y. Effects of the endoparasitoid Cotesia chilonis (Hymenoptera: Braconidae) parasitism, venom, and calyx fluid on cellular and humoral immunity of its host Chilo suppressalis (Lepidoptera: Crambidae) larvae. J. Insect Physiol. 2016, 85, 46–56. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvia, R.; Scieuzo, C.; Grimaldi, A.; Fanti, P.; Moretta, A.; Franco, A.; Varricchio, P.; Vinson, S.B.; Falabella, P. Role of Ovarian Proteins Secreted by Toxoneuron nigriceps (Viereck) (Hymenoptera, Braconidae) in the Early Suppression of Host Immune Response. Insects 2021, 12, 33. https://doi.org/10.3390/insects12010033
Salvia R, Scieuzo C, Grimaldi A, Fanti P, Moretta A, Franco A, Varricchio P, Vinson SB, Falabella P. Role of Ovarian Proteins Secreted by Toxoneuron nigriceps (Viereck) (Hymenoptera, Braconidae) in the Early Suppression of Host Immune Response. Insects. 2021; 12(1):33. https://doi.org/10.3390/insects12010033
Chicago/Turabian StyleSalvia, Rosanna, Carmen Scieuzo, Annalisa Grimaldi, Paolo Fanti, Antonio Moretta, Antonio Franco, Paola Varricchio, S. Bradleigh Vinson, and Patrizia Falabella. 2021. "Role of Ovarian Proteins Secreted by Toxoneuron nigriceps (Viereck) (Hymenoptera, Braconidae) in the Early Suppression of Host Immune Response" Insects 12, no. 1: 33. https://doi.org/10.3390/insects12010033