Oxidative Stress in Traumatic Brain Injury
Abstract
:1. Introduction
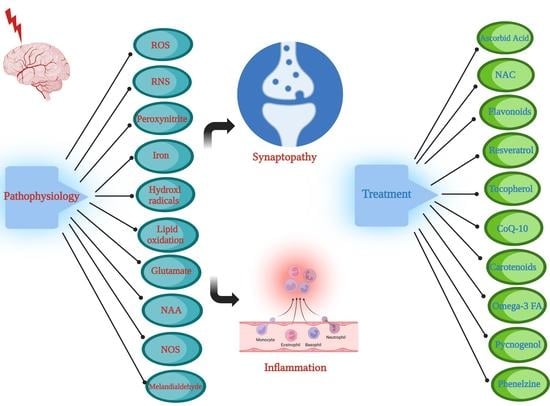
2. Pathophysiology of Oxidative Stress in TBI
2.1. Oxidative Enzymes
2.2. ROS and Oxidative Stress
2.3. RNS and Oxidative Stress
2.4. Iron and Hydroxyl Radicals
2.5. Lipid Peroxidation
2.6. Glutathione Peroxidase
2.7. Malondialdehyde
2.8. Glutamate
2.9. N-acetylaspartate
2.10. Nitric Oxide Synthase
3. Oxidative Stress and Synapse
4. Oxidative Stress and Inflammation
5. Treatment Options of Oxidative Stress
5.1. Ascorbic Acid (Vitamin C)
5.2. N-Acetyl-Cysteine (NAC)
5.3. Flavonoids
5.4. Resveratrol
5.5. Alpha-Tocopherol (Vitamin E)
5.6. Coenzyme Q10
5.7. Carotenoids
5.8. Omega-3 Fatty Acids
5.9. Pycnogenol
5.10. Phenelzine
| Therapeutic Agent | Mechanism | References |
|---|---|---|
| Ascorbic Acid (Vitamin C) | As a robust reducing agent, it reacts with a range of ROS and RNS. | Castiglione et al. 2018 [108] Parker et al. 2015 [112] |
| N-acetylcysteine (NAC) | direct role as ROS and RNS scavenging agent, or increasing cysteine availability and GSH synthesis, extensively studied in preclinical and clinical settings | Limongi et al. 2019 [115] Koza et al. 2019 [119] |
| Flavonoids | Given their negative oxido-reductive potential, flavonoids can function as robust anti-oxidants towards various ROS and RNS species | Izzi et al. 2012 [122] Theadom et al. 2013 [123] |
| Resveratrol | Has both anti-inflammatory benefits, as well as modulation of mitochondrial functions | Irias-Mata et al. 2017 [128] Gatson et al. 2013 [127] |
| a-Tocopherol (Vitamin E) | Fat soluble compounds, ubiquitous in vegetable oils, with potent anti-oxidant properties | Di Pietro et al. 2020 [97] Razmkon et al. 2011 [114] |
| Coenzyme Q10 | component of the mitochondrial chain aerobic organisms with efficacy in TBI treatment in a few preclinical studies | Pierce et al. 2017 [134] Pierce et al. 2018 [135] |
| Carotenoids | group of pigments synthesized by plants, algae, and species of bacteria, and in preclinical studies have bene shown to diminish apoptosis, and reduced expression of NF-κB-based inflammation | Zhang et al. 2017 [137] Ji et al. 2013 [139] |
| Omega-3 Fatty Acids | group of polyunsaturated fatty acids (PUFA), that are subdivided into essential or semi-essential for humans, and benefits of omega-3-fatty acids and DHA in preclinical TBI models | Matsuoka et al. 2015 [145] Wu et al. 2013 [146] |
| Pycnogenol (PYC) | combination of bioflavonoids with robust capacity to scavenge free radicals | Ansari et al. 2013 [147] |
| Phenelzine | FDA-approved drug, which functions as an MAO inhibitor and has been shown to have aldehyde scavenging properties, and provide partial protection from LP-mediated oxidative injury | Hill et al. 2020 [148] Cebak et al. 2017 [149] |
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eastman, C.L.; D’Ambrosio, R.; Ganesh, T. Modulating neuroinflammation and oxidative stress to prevent epilepsy and improve outcomes after traumatic brain injury. Neuropharmacology 2020, 172, 107907. [Google Scholar] [CrossRef] [PubMed]
- Leo, P.; Mccrea, M. Epidemiology. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2016; Chapter 1. [Google Scholar]
- Selassie, A.W.; Zaloshnja, E.; Langlois, J.A.; Miller, T.; Jones, P.; Steiner, C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 2008, 23, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-C.; Lin, Y.-J.; Shih, F.-Y.; Chang, H.-W.; Su, Y.-J.; Cheng, B.-C.; Su, C.-M.; Tsai, N.-W.; Chang, Y.-T.; Kwan, A.-L. The role of serial oxidative stress levels in acute traumatic brain injury and as predictors of outcome. World Neurosurg. 2016, 87, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic brain injury: An overview of epidemiology, pathophysiology, and medical management. Med. Clin. 2020, 104, 213–238. [Google Scholar]
- Pavlovic, D.; Pekic, S.; Stojanovic, M.; Popovic, V. Traumatic brain injury: Neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary 2019, 22, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.P.O.; Rodrigues, F.S.; Della-Pace, I.D.; Mota, B.C.; Oliveira, S.M.; de Campos Velho Gewehr, C.; Bobinski, F.; de Oliveira, C.V.; Brum, J.S.; Oliveira, M.S.; et al. The effect of NADPH-oxidase inhibitor apocynin on cognitive impairment induced by moderate lateral fluid percussion injury: Role of inflammatory and oxidative brain damage. Neurochem. Int. 2013, 63, 583–593. [Google Scholar] [CrossRef]
- Cornelius, C.; Crupi, R.; Calabrese, V.; Graziano, A.; Milone, P.; Pennisi, G.; Radak, Z.; Calabrese, E.J.; Cuzzocrea, S. Traumatic brain injury: Oxidative stress and neuroprotection. Antioxid. Redox Signal. 2013, 19, 836–853. [Google Scholar] [CrossRef]
- Greve, M.W.; Zink, B.J. Pathophysiology of traumatic brain injury. Mt. Sinai J. Med. J. Transl. Pers. Med. J. Transl. Pers. Med. 2009, 76, 97–104. [Google Scholar] [CrossRef]
- Herbert, V.; Shaw, S.; Jayatilleke, E.; Stopler-Kasdan, T. Most free-radical injury is iron-related: It is promoted by iron, hemin, holoferritin and vitamin c, and inhibited by desferoxamine and apoferritin. Stem Cells 1994, 12, 289–303. [Google Scholar] [CrossRef]
- Floyd, R.A.; Carney, J.M. Free radical damage to protein and DNA: Mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann. Neurol. 1992, 32, S22–S27. [Google Scholar] [CrossRef]
- Abdul-Muneer, P.; Chandra, N.; Haorah, J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 2015, 51, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Cordaro, M.; Cuzzocrea, S.; Impellizzeri, D. Management of traumatic brain injury: From present to future. Antioxidants 2020, 9, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordaro, M.; Trovato Salinaro, A.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Crea, R.; Cuzzocrea, S.; Di Paola, R.; et al. Hidrox® Roles in Neuroprotection: Biochemical Links between Traumatic Brain Injury and Alzheimer’s Disease. Antioxidants 2021, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.A.; Tajiri, N.; Sanberg, P.R.; Kaneko, Y.; Borlongan, C.V. Increased Amyloid Precursor Protein and Tau Expression Manifests as Key Secondary Cell Death in Chronic Traumatic Brain Injury. J. Cell. Physiol. 2017, 232, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Zhang, J.; Dong, J.-f.; Shi, F.-D. Dissemination of brain inflammation in traumatic brain injury. Cell. Mol. Immunol. 2019, 16, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Frati, A.; Cerretani, D.; Fiaschi, A.I.; Frati, P.; Gatto, V.; La Russa, R.; Pesce, A.; Pinchi, E.; Santurro, A.; Fraschetti, F. Diffuse axonal injury and oxidative stress: A comprehensive review. Int. J. Mol. Sci. 2017, 18, 2600. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.-Y.; Wang, H.-D.; Xu, J.-G.; Ding, K.; Li, T. NADPH oxidase inhibition improves neurological outcome in experimental traumatic brain injury. Neurochem. Int. 2014, 69, 14–19. [Google Scholar] [CrossRef]
- Ansari, M.A.; Roberts, K.N.; Scheff, S.W. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic. Biol. Med. 2008, 45, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Bayir, H.; Kagan, V.E.; Tyurina, Y.Y.; Tyurin, V.; Ruppel, R.A.; Adelson, P.D.; Graham, S.H.; Janesko, K.; Clark, R.S.; Kochanek, P.M. Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr. Res. 2002, 51, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Hall, E.D.; Detloff, M.R.; Johnson, K.; Kupina, N.C. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J. Neurotrauma 2004, 21, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Aravind, A.; Pfister, B.J.; Chandra, N.; Haorah, J. Animal Models of Traumatic Brain Injury and Assessment of Injury Severity. Mol. Neurobiol. 2019, 56, 5332–5345. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CERNAK, I.; SAVIC, V.J.; KOTUR, J.; PROKIC, V.; VELJOVIC, M.; GRBOVIC, D. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J. Neurotrauma 2000, 17, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.; Yonkers, P.; Andrus, P.; Cox, J.; Anderson, D. Biochemistry and pharmacology of lipid antioxidants in acute brain and spinal cord injury. J. Neurotrauma 1992, 9, S425–S442. [Google Scholar] [PubMed]
- Ano, Y.; Sakudo, A.; Kimata, T.; Uraki, R.; Sugiura, K.; Onodera, T. Oxidative damage to neurons caused by the induction of microglial NADPH oxidase in encephalomyocarditis virus infection. Neurosci. Lett. 2010, 469, 39–43. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Balazy, M.; Nigam, S. Aging, lipid modifications and phospholipases—New concepts. Ageing Res. Rev. 2003, 2, 191–209. [Google Scholar] [CrossRef]
- Brandes, R.P.; Weissmann, N.; Schröder, K. NADPH oxidases in cardiovascular disease. Free Radic. Biol. Med. 2010, 49, 687–706. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Andrew, P.J.; Mayer, B. Enzymatic function of nitric oxide synthases. Cardiovasc. Res. 1999, 43, 521–531. [Google Scholar] [CrossRef]
- Hall, E.D.; Wang, J.A.; Miller, D.M. Relationship of nitric oxide synthase induction to peroxynitrite-mediated oxidative damage during the first week after experimental traumatic brain injury. Exp. Neurol. 2012, 238, 176–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mésenge, C.; Verrecchia, C.; Allix, M.; Boulu, R.R.; Plotkine, M. Reduction of the neurological deficit in mice with traumatic brain injury by nitric oxide synthase inhibitors. J. Neurotrauma 1996, 13, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D.; Vaishnav, R.A.; Mustafa, A.G. Antioxidant therapies for traumatic brain injury. Neurotherapeutics 2010, 7, 51–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daglas, M.; Adlard, P.A. The involvement of iron in traumatic brain injury and neurodegenerative disease. Front. Neurosci. 2018, 12, 981. [Google Scholar] [CrossRef] [Green Version]
- Zaleska, M.M.; Floyd, R.A. Regional lipid peroxidation in rat brain in vitro: Possible role of endogenous iron. Neurochem. Res 1985, 10, 397–410. [Google Scholar] [CrossRef]
- Halliwell, B. The wanderings of a free radical. Free Radic. Biol. Med. 2009, 46, 531–542. [Google Scholar] [CrossRef]
- Sadrzadeh, S.M.; Eaton, J.W. Hemoglobin-mediated oxidant damage to the central nervous system requires endogenous ascorbate. J. Clin. Investig. 1988, 82, 1510–1515. [Google Scholar] [CrossRef] [Green Version]
- Andersen, H.H.; Johnsen, K.B.; Moos, T. Iron deposits in the chronically inflamed central nervous system and contributes to neurodegeneration. Cell. Mol. Life Sci. 2014, 71, 1607–1622. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Tan, L.; Li, H.; Zhang, Q.; Li, Y.; Guo, J. Deferoxamine therapy for intracerebral hemorrhage: A systematic review. PLoS ONE 2018, 13, e0193615. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Okauchi, M.; Hua, Y.; Keep, R.F.; Xi, G. Deferoxamine reduces neuronal death and hematoma lysis after intracerebral hemorrhage in aged rats. Transl. Stroke Res. 2013, 4, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Okatani, Y.; Wakatsuki, A.; Kaneda, C. Melatonin increases activities of glutathione peroxidase and superoxide dismutase in fetal rat brain. J. Pineal Res. 2000, 28, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Povlishock, J.; Kontos, H. The role of oxygen radicals in the pathobiology of traumatic brain injury. Hum. Cell 1992, 5, 345–353. [Google Scholar]
- Kushner, D. Mild traumatic brain injury: Toward understanding manifestations and treatment. Arch. Intern. Med. 1998, 158, 1617–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedel, G.; Platt, B.; Micheau, J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003, 140, 1–47. [Google Scholar] [CrossRef]
- Yi, J.H.; Hazell, A.S. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem. Int. 2006, 48, 394–403. [Google Scholar] [CrossRef]
- Friedman, S.D.; Brooks, W.M.; Jung, R.E.; Chiulli, S.J.; Sloan, J.H.; Montoya, B.T.; Hart, B.L.; Yeo, R.A. Quantitative proton MRS predicts outcome after traumatic brain injury. Neurology 1999, 52, 1384–1391. [Google Scholar] [CrossRef]
- Signoretti, S.; Marmarou, A.; Tavazzi, B.; Lazzarino, G.; Beaumont, A.; Vagnozzi, R. N-Acetylaspartate reduction as a measure of injury severity and mitochondrial dysfunction following diffuse traumatic brain injury. J. Neurotrauma 2001, 18, 977–991. [Google Scholar] [CrossRef]
- Tavazzi, B.; Vagnozzi, R.; Di Pierro, D.; Amorini, A.M.; Fazzina, G.; Signoretti, S.; Marmarou, A.; Caruso, I.; Lazzarino, G. Ion-pairing high-performance liquid chromatographic method for the detection of N-acetylaspartate and N-acetylglutamate in cerebral tissue extracts. Anal. Biochem. 2000, 277, 104–108. [Google Scholar] [CrossRef]
- Wada, K.; Chatzipanteli, K.; Busto, R.; Dietrich, W.D. Role of nitric oxide in traumatic brain injury in the rat. J. Neurosurg. 1998, 89, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Cherian, L.; Hlatky, R.; Robertson, C.S. Nitric oxide in traumatic brain injury. Brain Pathol. 2004, 14, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-J.; Sherwood, E.R.; Prough, D.S.; Yie Lin, C.; DeWitt, D.S. The effects of traumatic brain injury on cerebral blood flow and brain tissue nitric oxide levels and cytokine expression. J. Neurotrauma 2004, 21, 1431–1442. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Bahrami, S.; Redl, H.; Szabo, C. Alterations in nitric oxide homeostasis during traumatic brain injury. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 2627–2632. [Google Scholar] [CrossRef] [PubMed]
- Besson, V.C. Drug targets for traumatic brain injury from poly (ADP-ribose) polymerase pathway modulation. Br. J. Pharmacol. 2009, 157, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, A.; Trescher, W.H.; Lange, M.S.; Johnston, M.V. Prolonged suppression of brain nitric oxide synthase activity by 7-nitroindazole protects against cerebral hypoxic–ischemic injury in neonatal rat. Brain Dev. 2001, 23, 349–354. [Google Scholar] [CrossRef]
- Hall, E.D.; Andrus, P.K.; Yonkers, P.A. Brain hydroxyl radical generation in acute experimental head injury. J. Neurochem. 1993, 60, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Tyurin, V.A.; Tyurina, Y.Y.; Borisenko, G.G.; Sokolova, T.V.; Ritov, V.B.; Quinn, P.J.; Rose, M.; Kochanek, P.; Graham, S.H.; Kagan, V.E. Oxidative stress following traumatic brain injury in rats: Quantitation of biomarkers and detection of free radical intermediates. J. Neurochem. 2000, 75, 2178–2189. [Google Scholar] [CrossRef] [Green Version]
- Licastro, F.; Hrelia, S.; Porcellini, E.; Malaguti, M.; Di Stefano, C.; Angeloni, C.; Carbone, I.; Simoncini, L.; Piperno, R. Peripheral inflammatory markers and antioxidant response during the post-acute and chronic phase after severe traumatic brain injury. Front. Neurol. 2016, 7, 189. [Google Scholar] [CrossRef] [Green Version]
- Jamjoom, A.A.; Rhodes, J.; Andrews, P.J.; Grant, S.G. The synapse in traumatic brain injury. Brain 2021, 144, 18–31. [Google Scholar] [CrossRef]
- Szydlowska, K.; Tymianski, M. Calcium, ischemia and excitotoxicity. Cell Calcium 2010, 47, 122–129. [Google Scholar] [CrossRef]
- Brenman, J.E.; Chao, D.S.; Gee, S.H.; McGee, A.W.; Craven, S.E.; Santillano, D.R.; Wu, Z.; Huang, F.; Xia, H.; Peters, M.F.; et al. Interaction of Nitric Oxide Synthase with the Postsynaptic Density Protein PSD-95 and α1-Syntrophin Mediated by PDZ Domains. Cell 1996, 84, 757–767. [Google Scholar] [CrossRef] [Green Version]
- Bach, A.; Clausen, B.H.; Kristensen, L.K.; Andersen, M.G.; Ellman, D.G.; Hansen, P.B.L.; Hasseldam, H.; Heitz, M.; Özcelik, D.; Tuck, E.J.; et al. Selectivity, efficacy and toxicity studies of UCCB01-144, a dimeric neuroprotective PSD-95 inhibitor. Neuropharmacology 2019, 150, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Boutté, A.; Yu, X.; Dutta, B.; Feala, J.D.; Schmid, K.; Dave, J.; Tawa, G.J.; Wallqvist, A.; Reifman, J. A systems biology strategy to identify molecular mechanisms of action and protein indicators of traumatic brain injury. J. Neurosci. Res. 2015, 93, 199–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, M.A.; Roberts, K.N.; Scheff, S.W. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J. Neurotrauma 2008, 25, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Bullock, R.; Zauner, A.; Woodward, J.J.; Myseros, J.; Choi, S.C.; Ward, J.D.; Marmarou, A.; Young, H.F. Factors affecting excitatory amino acid release following severe human head injury. J. Neurosurg. 1998, 89, 507–518. [Google Scholar] [CrossRef]
- Palmer, A.M.; Marion, D.W.; Botscheller, M.L.; Swedlow, P.E.; Styren, S.D.; DeKosky, S.T. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J. Neurochem. 1993, 61, 2015–2024. [Google Scholar] [CrossRef]
- Faden, A.I.; Demediuk, P.; Panter, S.S.; Vink, R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 1989, 244, 798–800. [Google Scholar] [CrossRef]
- Yi, J.-H.; Hoover, R.; McIntosh, T.K.; Hazell, A.S. Early, transient increase in complexin I and complexin II in the cerebral cortex following traumatic brain injury is attenuated by N-acetylcysteine. J. Neurotrauma 2006, 23, 86–96. [Google Scholar] [CrossRef]
- Koizumi, H.; Fujisawa, H.; Ito, H.; Maekawa, T.; Di, X.; Bullock, R. Effects of mild hypothermia on cerebral blood flow-independent changes in cortical extracellular levels of amino acids following contusion trauma in the rat. Brain Res. 1997, 747, 304–312. [Google Scholar] [CrossRef]
- Yi, J.-H.; Hazell, A.S. N-acetylcysteine attenuates early induction of heme oxygenase-1 following traumatic brain injury. Brain Res. 2005, 1033, 13–19. [Google Scholar] [CrossRef]
- Pabst, S.; Margittai, M.; Vainius, D.; Langen, R.; Jahn, R.; Fasshauer, D. Rapid and selective binding to the synaptic SNARE complex suggests a modulatory role of complexins in neuroexocytosis. J. Biol. Chem. 2002, 277, 7838–7848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eastwood, S.; Cotter, D.; Harrison, P. Cerebellar synaptic protein expression in schizophrenia. Neuroscience 2001, 105, 219–229. [Google Scholar] [CrossRef]
- Redell, J.B.; Moore, A.N.; Dash, P.K. Expression of the prodynorphin gene after experimental brain injury and its role in behavioral dysfunction. Exp. Biol. Med. 2003, 228, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006, 147, S232–S240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, C.; Engelhard, K. Pathophysiology of traumatic brain injury. BJA Br. J. Anaesth. 2007, 99, 4–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hans, V.H.; Kossmann, T.; Joller, H.; Otto, V.; Morganti-Kossmann, M.-C. Interleukin-6 and its soluble receptor in serum and cerebrospinal fluid after cerebral trauma. Neuroreport 1999, 10, 409–412. [Google Scholar] [CrossRef]
- Soares, H.D.; Hicks, R.R.; Smith, D.; McIntosh, T.K. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J. Neurosci. 1995, 15, 8223–8233. [Google Scholar] [CrossRef] [Green Version]
- Vecil, G.G.; Larsen, P.H.; Corley, S.M.; Herx, L.M.; Besson, A.; Goodyer, C.G.; Yong, V.W. Interleukin-1 is a key regulator of matrix metalloproteinase-9 expression in human neurons in culture and following mouse brain trauma in vivo. J. Neurosci. Res. 2000, 61, 212–224. [Google Scholar] [CrossRef]
- Lawrence, C.B.; Allan, S.M.; Rothwell, N.J. Interleukin-1β and the interleukin-1 receptor antagonist act in the striatum to modify excitotoxic brain damage in the rat. Eur. J. Neurosci. 1998, 10, 1188–1195. [Google Scholar] [CrossRef]
- Hopkins, S.J.; Rothwell, N.J. Cytokines and the nervous system I: Expression and recognition. Trends Neurosci. 1995, 18, 83–88. [Google Scholar] [CrossRef]
- Rothwell, N.J.; Hopkins, S.J. Cytokines and the nervous system II: Actions and mechanisms of action. Trends Neurosci. 1995, 18, 130–136. [Google Scholar] [CrossRef]
- Lu, K.-T.; Wang, Y.-W.; Yang, J.-T.; Yang, Y.-L.; Chen, H.-I. Effect of interleukin-1 on traumatic brain injury–induced damage to hippocampal neurons. J. Neurotrauma 2005, 22, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Knoblach, S.M.; Faden, A.I. Cortical interleukin-1β elevation after traumatic brain injury in the rat: No effect of two selective antagonists on motor recovery. Neurosci. Lett. 2000, 289, 5–8. [Google Scholar] [CrossRef]
- Clausen, F.; Hånell, A.; Israelsson, C.; Hedin, J.; Ebendal, T.; Mir, A.K.; Gram, H.; Marklund, N. Neutralization of interleukin-1β reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur. J. Neurosci. 2011, 34, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Csuka, E.; Morganti-Kossmann, M.C.; Lenzlinger, P.M.; Joller, H.; Trentz, O.; Kossmann, T. IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: Relationship to IL-6, TNF-α, TGF-β1 and blood–brain barrier function. J. Neuroimmunol. 1999, 101, 211–221. [Google Scholar] [CrossRef]
- Ross, S.A.; Halliday, M.I.; Campbell, G.C.; Byrnes, D.P.; Rowlands, B.J. The presence of tumour necrosis factor in CSF and plasma after severe head injury. Br. J. Neurosurg. 1994, 8, 419–425. [Google Scholar] [CrossRef]
- Khuman, J.; Meehan III, W.P.; Zhu, X.; Qiu, J.; Hoffmann, U.; Zhang, J.; Giovannone, E.; Lo, E.H.; Whalen, M.J. Tumor necrosis factor alpha and Fas receptor contribute to cognitive deficits independent of cell death after concussive traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 2011, 31, 778–789. [Google Scholar] [CrossRef] [Green Version]
- Berrrrpohl, D.; You, Z.; Lo, E.H.; Kim, H.-H.; Whalen, M.J. TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 2007, 27, 1806–1818. [Google Scholar] [CrossRef] [Green Version]
- Rimaniol, A.-C.; Lekieffre, D.; Serrano, A.; Masson, A.; Benavides, J.; Zavala, F. Biphasic transforming growth factor-beta production flanking the pro-inflammatory cytokine response in cerebral trauma. Neuroreport 1995, 7, 133–136. [Google Scholar] [CrossRef]
- Morganti-Kossmann, M.C.; Rancan, M.; Stahel, P.F.; Kossmann, T. Inflammatory response in acute traumatic brain injury: A double-edged sword. Curr. Opin. Crit. Care 2002, 8, 101–105. [Google Scholar] [CrossRef]
- Cacheaux, L.P.; Ivens, S.; David, Y.; Lakhter, A.J.; Bar-Klein, G.; Shapira, M.; Heinemann, U.; Friedman, A.; Kaufer, D. Transcriptome profiling reveals TGF-β signaling involvement in epileptogenesis. J. Neurosci. 2009, 29, 8927–8935. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, G.; Zhang, J.; Strong, R.; Dash, P.K.; Kan, Y.W.; Grotta, J.C.; Aronowski, J. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke 2007, 38, 3280–3286. [Google Scholar] [CrossRef] [PubMed]
- Casili, G.; Campolo, M.; Paterniti, I.; Lanza, M.; Filippone, A.; Cuzzocrea, S.; Esposito, E. Dimethyl fumarate attenuates neuroinflammation and neurobehavioral deficits induced by experimental traumatic brain injury. J. Neurotrauma 2018, 35, 1437–1451. [Google Scholar] [CrossRef]
- Jin, W.; Wang, H.-D.; Hu, Z.-g.; Yan, W.; Chen, G.; Yin, H.-X. Transcription factor Nrf2 plays a pivotal role in protection against traumatic brain injury-induced acute intestinal mucosal injury in mice. J. Surg. Res. 2009, 157, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.-G.; Zhang, G.-D.; Shi, P.-Q.; Du, B.-S. Expression and antioxidation of Nrf2/ARE pathway in traumatic brain injury. Asian Pac. J. Trop. Med. 2013, 6, 305–310. [Google Scholar] [CrossRef]
- Di Pietro, V.; Yakoub, K.M.; Caruso, G.; Lazzarino, G.; Signoretti, S.; Barbey, A.K.; Tavazzi, B.; Lazzarino, G.; Belli, A.; Amorini, A.M. Antioxidant therapies in traumatic brain injury. Antioxidants 2020, 9, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzarino, G.; Listorti, I.; Bilotta, G.; Capozzolo, T.; Amorini, A.M.; Longo, S.; Caruso, G.; Lazzarino, G.; Tavazzi, B.; Bilotta, P. Water- and Fat-Soluble Antioxidants in Human Seminal Plasma and Serum of Fertile Males. Antioxidants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Gregorio, R.; Simal-Gandara, J. A critical review of bioactive food components, and of their functional mechanisms, biological effects and health outcomes. Curr. Pharm. Des. 2017, 23, 2731–2741. [Google Scholar] [CrossRef]
- Rigg, J.L.; Elovic, E.P.; Greenwald, B.D. A Review of the Effectiveness of Antioxidant Therapy to Reduce Neuronal Damage in Acute Traumatic Brain Injury. J. Head Trauma Rehabil. 2005, 20, 389–391. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Taffe, K.M.; Abrahamson, E.E.; Dixon, C.E.; Kochanek, P.M.; Ikonomovic, M.D. Time course analysis of hippocampal nerve growth factor and antioxidant enzyme activity following lateral controlled cortical impact brain injury in the rat. J. Neurotrauma 2004, 21, 491–500. [Google Scholar] [CrossRef]
- Veronese, F.M.; Caliceti, P.; Schiavon, O.; Sergi, M. Polyethylene glycol–superoxide dismutase, a conjugate in search of exploitation. Adv. Drug Deliv. Rev. 2002, 54, 587–606. [Google Scholar] [CrossRef]
- Ding, K.; Wang, H.; Xu, J.; Li, T.; Zhang, L.; Ding, Y.; Zhu, L.; He, J.; Zhou, M. Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: The Nrf2–ARE signaling pathway as a potential mechanism. Free Radic. Biol. Med. 2014, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, N.; Katayama, Y.; Kawamata, T.; Maeda, T.; Mori, T.; Yamamoto, T.; Kikuchi, T.; Uwahodo, Y. Effects of antioxidant, OPC-14117, on secondary cellular damage and behavioral deficits following cortical contusion in the rat. Brain Res. 2002, 934, 117–124. [Google Scholar] [CrossRef]
- Almli, L.M.; Hamrick, S.E.; Koshy, A.A.; Täuber, M.G.; Ferriero, D.M. Multiple pathways of neuroprotection against oxidative stress and excitotoxic injury in immature primary hippocampal neurons. Dev. Brain Res. 2001, 132, 121–129. [Google Scholar] [CrossRef]
- Granger, M.; Eck, P. Dietary vitamin C in human health. Adv. Food Nutr. Res. 2018, 83, 281–310. [Google Scholar]
- Hirschmann, J.; Raugi, G.J. Adult scurvy. J. Am. Acad. Dermatol. 1999, 41, 895–910. [Google Scholar] [CrossRef]
- Castiglione, D.; Platania, A.; Conti, A.; Falla, M.; D’Urso, M.; Marranzano, M. Dietary Micronutrient and Mineral Intake in the Mediterranean Healthy Eating, Ageing, and Lifestyle (MEAL) Study. Antioxidants 2018, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Bürzle, M.; Suzuki, Y.; Ackermann, D.; Miyazaki, H.; Maeda, N.; Clémençon, B.; Burrier, R.; Hediger, M.A. The sodium-dependent ascorbic acid transporter family SLC23. Mol. Asp. Med. 2013, 34, 436–454. [Google Scholar] [CrossRef]
- Di Pietro, V.; Lazzarino, G.; Amorini, A.M.; Tavazzi, B.; D’Urso, S.; Longo, S.; Vagnozzi, R.; Signoretti, S.; Clementi, E.; Giardina, B.; et al. Neuroglobin expression and oxidant/antioxidant balance after graded traumatic brain injury in the rat. Free Radic. Biol. Med. 2014, 69, 258–264. [Google Scholar] [CrossRef]
- Grünewald, R.A. Ascorbic acid in the brain. Brain Res. Rev. 1993, 18, 123–133. [Google Scholar] [CrossRef]
- Parker, W.H.; Qu, Z.-c.; May, J.M. Ascorbic acid transport in brain microvascular pericytes. Biochem. Biophys. Res. Commun. 2015, 458, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavazzi, B.; Signoretti, S.; Lazzarino, G.; Amorini, A.M.; Delfini, R.; Cimatti, M.; Marmarou, A.; Vagnozzi, R. Cerebral oxidative stress and depression of energy metabolism correlate with severity of diffuse brain injury in rats. Neurosurgery 2005, 56, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Razmkon, A.; Sadidi, A.; Sherafat-Kazemzadeh, E.; Mehrafshan, A.; Jamali, M.; Malekpour, B.; Saghafinia, M. Administration of vitamin C and vitamin E in severe head injury: A randomized double-blind controlled trial. Neurosurgery 2011, 58, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Limongi, D.; Baldelli, S.; Checconi, P.; Marcocci, M.E.; De Chiara, G.; Fraternale, A.; Magnani, M.; Ciriolo, M.R.; Palamara, A.T. GSH-C4 Acts as Anti-inflammatory Drug in Different Models of Canonical and Cell Autonomous Inflammation through NFκB Inhibition. Front. Immunol. 2019, 10, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steullet, P.; Neijt, H.C.; Cuénod, M.; Do, K.Q. Synaptic plasticity impairment and hypofunction of NMDA receptors induced by glutathione deficit: Relevance to schizophrenia. Neuroscience 2006, 137, 807–819. [Google Scholar] [CrossRef]
- Varga, V.; Jenei, Z.; Janáky, R.; Saransaari, P.; Oja, S.S. Glutathione is an endogenous ligand of rat brain N-methyl-D-aspartate (NMDA) and 2-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors. Neurochem. Res. 1997, 22, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Reed, T.T.; Owen, J.; Pierce, W.M.; Sebastian, A.; Sullivan, P.G.; Butterfield, D.A. Proteomic identification of nitrated brain proteins in traumatic brain-injured rats treated postinjury with gamma-glutamylcysteine ethyl ester: Insights into the role of elevation of glutathione as a potential therapeutic strategy for traumatic brain injury. J. Neurosci. Res. 2009, 87, 408–417. [Google Scholar] [CrossRef]
- Koza, L.; Linseman, D.A. Glutathione precursors shield the brain from trauma. Neural Regen. Res. 2019, 14, 1701–1702. [Google Scholar] [CrossRef]
- Sangobowale, M.; Nikulina, E.; Bergold, P.J. Minocycline plus N-acetylcysteine protect oligodendrocytes when first dosed 12 hours after closed head injury in mice. Neurosci. Lett. 2018, 682, 16–20. [Google Scholar] [CrossRef]
- Bhatti, J.; Nascimento, B.; Akhtar, U.; Rhind, S.G.; Tien, H.; Nathens, A.; da Luz, L.T. Systematic Review of Human and Animal Studies Examining the Efficacy and Safety of N-Acetylcysteine (NAC) and N-Acetylcysteine Amide (NACA) in Traumatic Brain Injury: Impact on Neurofunctional Outcome and Biomarkers of Oxidative Stress and Inflammation. Front. Neurol. 2018, 8, 744. [Google Scholar] [CrossRef] [Green Version]
- Izzi, V.; Masuelli, L.; Tresoldi, I.; Sacchetti, P.; Modesti, A.; Galvano, F.; Bei, R. The effects of dietary flavonoids on the regulation of redox inflammatory networks. Front. Biosci.-Landmark 2012, 17, 2396–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theadom, A.; Mahon, S.; Barker-Collo, S.; McPherson, K.; Rush, E.; Vandal, A.C.; Feigin, V.L. Enzogenol for cognitive functioning in traumatic brain injury: A pilot placebo-controlled RCT. Eur. J. Neurol. 2013, 20, 1135–1144. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Ho, C.-T.; Chen, Y.-K. Biological actions and molecular effects of resveratrol, pterostilbene, and 3′-hydroxypterostilbene. J. Food Drug Anal. 2017, 25, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Ates, O.; Cayli, S.; Altinoz, E.; Gurses, I.; Yucel, N.; Sener, M.; Kocak, A.; Yologlu, S. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol. Cell. Biochem. 2007, 294, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Chen, T.H.; Yang, L.Y.; Shih, C.M. Resveratrol protects astrocytes against traumatic brain injury through inhibiting apoptotic and autophagic cell death. Cell Death Dis. 2014, 5, e1147. [Google Scholar] [CrossRef] [Green Version]
- Gatson, J.W.; Liu, M.-M.; Abdelfattah, K.; Wigginton, J.G.; Smith, S.; Wolf, S.; Minei, J.P. Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J. Trauma Acute Care Surg. 2013, 74, 470–475. [Google Scholar] [CrossRef]
- Irías-Mata, A.; Stuetz, W.; Sus, N.; Hammann, S.; Gralla, K.; Cordero-Solano, A.; Vetter, W.; Frank, J. Tocopherols, Tocomonoenols, and Tocotrienols in Oils of Costa Rican Palm Fruits: A Comparison between Six Varieties and Chemical versus Mechanical Extraction. J. Agric. Food Chem. 2017, 65, 7476–7482. [Google Scholar] [CrossRef]
- Inci, S.; Özcan, O.E.; Kilinç, K. Time-Level Relationship for Lipid Peroxidation and the Protective Effect of α-Tocopherol in Experimental Mild and Severe Brain Injury. Neurosurgery 1998, 43, 330–335. [Google Scholar] [CrossRef]
- Yang, J.; Han, Y.; Ye, W.; Liu, F.; Zhuang, K.; Wu, G. Alpha tocopherol treatment reduces the expression of Nogo-A and NgR in rat brain after traumatic brain injury. J. Surg. Res. 2013, 182, e69–e77. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Vitamin E Protects Against Oxidative Damage and Learning Disability After Mild Traumatic Brain Injury in Rats. Neurorehabil. Neural Repair 2010, 24, 290–298. [Google Scholar] [CrossRef] [Green Version]
- Jorat, M.V.; Tabrizi, R.; Kolahdooz, F.; Akbari, M.; Salami, M.; Heydari, S.T.; Asemi, Z. The effects of coenzyme Q10 supplementation on biomarkers of inflammation and oxidative stress in among coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology 2019, 27, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, M.; Unal, M.M.; Gul, S.; Acikgoz, S.; Kandemir, N.; Hanci, V.; Edebali, N.; Acikgoz, B. Effect of Coenzyme Q10 on ischemia and neuronal damage in an experimental traumatic brain-injury model in rats. BMC Neurosci. 2011, 12, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, J.D.; Shen, Q.; Peltzer, J.; Thimmesch, A.; Hiebert, J.B. A pilot study exploring the effects of ubiquinol on brain genomics after traumatic brain injury. Nurs. Outlook 2017, 65, S44–S52. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.D.; Gupte, R.; Thimmesch, A.; Shen, Q.; Hiebert, J.B.; Brooks, W.M.; Clancy, R.L.; Diaz, F.J.; Harris, J.L. Ubiquinol treatment for TBI in male rats: Effects on mitochondrial integrity, injury severity, and neurometabolism. J. Neurosci. Res. 2018, 96, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Shin, M.; Kim, S.; Lee, S.B. Recent Advances in Studies on the Therapeutic Potential of Dietary Carotenoids in Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2018, 2018, 4120458. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, Z.; Cui, H.; Wang, Y.; Zhong, C. Astaxanthin protects astrocytes against trauma-induced apoptosis through inhibition of NKCC1 expression via the NF-κB signaling pathway. BMC Neurosci. 2017, 18, 42. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Cui, Z.; Cui, H.; Cao, Y.; Wang, Y.; Zhong, C. Astaxanthin alleviates cerebral edema by modulating NKCC1 and AQP4 expression after traumatic brain injury in mice. BMC Neurosci. 2016, 17, 60. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Peng, D.; Zhang, Y.; Zhang, J.; Wang, Y.; Gao, Y.; Lu, N.; Tang, P. Astaxanthin improves cognitive performance in mice following mild traumatic brain injury. Brain Res. 2017, 1659, 88–95. [Google Scholar] [CrossRef]
- Zhong, J.; Jiang, L.; Huang, Z.; Zhang, H.; Cheng, C.; Liu, H.; He, J.; Wu, J.; Darwazeh, R.; Wu, Y.; et al. The long non-coding RNA Neat1 is an important mediator of the therapeutic effect of bexarotene on traumatic brain injury in mice. Brain Behav. Immun. 2017, 65, 183–194. [Google Scholar] [CrossRef]
- Zhong, J.; Cheng, C.; Liu, H.; Huang, Z.; Wu, Y.; Teng, Z.; He, J.; Zhang, H.; Wu, J.; Cao, F.; et al. Bexarotene protects against traumatic brain injury in mice partially through apolipoprotein E. Neuroscience 2017, 343, 434–448. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, L.; Rao, W.; Su, N.; Hui, H.; Wang, L.; Peng, C.; Tu, Y.; Zhang, S.; Fei, Z. Neuroprotective effects of crocin against traumatic brain injury in mice: Involvement of notch signaling pathway. Neurosci. Lett. 2015, 591, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Yu, X.; Chen, M.; Chen, J.; Xu, J. Lutein protects against severe traumatic brain injury through anti-inflammation and antioxidative effects via ICAM-1/Nrf-2. Mol. Med. Rep. 2017, 16, 4235–4240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauritzen, L.; Hansen, H.S.; Jørgensen, M.H.; Michaelsen, K.F. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 2001, 40, 1–94. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Nishi, D.; Tanima, Y.; Itakura, M.; Kojima, M.; Hamazaki, K.; Noguchi, H.; Hamazaki, T. Serum pro-BDNF/BDNF as a treatment biomarker for response to docosahexaenoic acid in traumatized people vulnerable to developing psychological distress: A randomized controlled trial. Transl. Psychiatry 2015, 5, e596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Exercise facilitates the action of dietary DHA on functional recovery after brain trauma. Neuroscience 2013, 248, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.A.; Roberts, K.N.; Scheff, S.W. Dose-and time-dependent neuroprotective effects of Pycnogenol® following traumatic brain injury. J. Neurotrauma 2013, 30, 1542–1549. [Google Scholar] [CrossRef] [Green Version]
- Hill, R.L.; Singh, I.N.; Wang, J.A.; Kulbe, J.R.; Hall, E.D. Protective effects of phenelzine administration on synaptic and non-synaptic cortical mitochondrial function and lipid peroxidation-mediated oxidative damage following TBI in young adult male rats. Exp. Neurol. 2020, 330, 113322. [Google Scholar] [CrossRef]
- Cebak, J.E.; Singh, I.N.; Hill, R.L.; Wang, J.A.; Hall, E.D. Phenelzine protects brain mitochondrial function in vitro and in vivo following traumatic brain injury by scavenging the reactive carbonyls 4-hydroxynonenal and acrolein leading to cortical histological neuroprotection. J. Neurotrauma 2017, 34, 1302–1317. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.N.; Gilmer, L.K.; Miller, D.M.; Cebak, J.E.; Wang, J.A.; Hall, E.D. Phenelzine Mitochondrial Functional Preservation and Neuroprotection after Traumatic Brain Injury Related to Scavenging of the Lipid Peroxidation-Derived Aldehyde 4-Hydroxy-2-Nonenal. J. Cereb. Blood Flow Metab. 2013, 33, 593–599. [Google Scholar] [CrossRef]

| Pathological Agent | Mechanism | References |
|---|---|---|
| Reactive Oxygen Species (ROS) | ROS includes superoxide (O2●−), hydrogen peroxide (H2O2), and the hydroxyl radical (●OH), produced by mitochondria and product of cellular oxidative metabolism | Eastman et al. 2020 [1] Pisochi et al. 2015 [24] |
| Reactive Nitrogen Species (RNS) | RNS Includes nitric oxide compounds, such as peroxynitrie (ONOO−), and nitrogen dioxide (NO2), and have longer half-lives than ROS | Balazy et al. 2003 [25] Brandes et al. 2010 [26] Uttara et al. 2009 [27] |
| Peroxynitrite (PN) | PN can also react with carbon dioxide (CO2), and form nitoperoxocarbonate (ONOOCO2) & subsequent cellular damage via transfer of electron from hydrogen atom bound to allylic carbon in polyunsaturated fatty acids (PUFAs) | Hall et al. 2012 [28] Hall et al. 2004 [21] |
| Iron and Hydroxyl Radicals | Post TBI, the PH within injured areas are lowered, in turn leading to release of iron from its storage sites with an additional source of catalytically active iron being hemoglobin, in turn responsible for hemoglobin mediated oxidative damage | Daglas et al. 2018 [29] Hatakeyama et al. 2013 [30] Zeng et al. 2018 [31] |
| Lipid Peroxidation (LP) | LP has been associated with loss of cell membrane integrity, increased membrane permeability, diminished activity in membrane ATPase, in turn leading to further injury | Cornelius et al. 2013 [8] |
| Glutathione Peroxidase (GPx) | GPx reactions lead to oxidation of glutathione (GSH) to oxidized form (GSSG) | Kushner et al. 1998 [32] |
| Malondialdehyde (MDA) | MDA reacts with several functional groups on proteins, lipoproteins and DNA, leading to further oxidative damage | Cornelius et al. 2013 [8] |
| Glutamate | TBI involves excessive release of extracellular glutamate, overstimulation of metabotropic and ionotropic receptors, and neuronal cell death | Riedel at al. 2003 [33] Yi et al. 2006 [34] |
| N-acetylaspartate (NAA) | N-acetylaspartate (NAA) is the acetylated form of aspartate, ubiquitous in CNS and a robust injury marker | Signoretti et al. 2001 [35] Tavazzi et al. 2000 [36] |
| Nitric oxide synthase (NOS) | TBI leads to activation of NOS, in turn the formation of NO in the brain | Besson et al. 2009 [37] Ishida et al. 2001 [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fesharaki-Zadeh, A. Oxidative Stress in Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 13000. https://doi.org/10.3390/ijms232113000
Fesharaki-Zadeh A. Oxidative Stress in Traumatic Brain Injury. International Journal of Molecular Sciences. 2022; 23(21):13000. https://doi.org/10.3390/ijms232113000
Chicago/Turabian StyleFesharaki-Zadeh, Arman. 2022. "Oxidative Stress in Traumatic Brain Injury" International Journal of Molecular Sciences 23, no. 21: 13000. https://doi.org/10.3390/ijms232113000