An Overview: The Toxicity of Ageratina adenophora on Animals and Its Possible Interventions
Abstract
:1. Introduction
2. Invasive Nature of A. adenophora
3. Major Toxins in A. adenophora and Their Toxic Nature
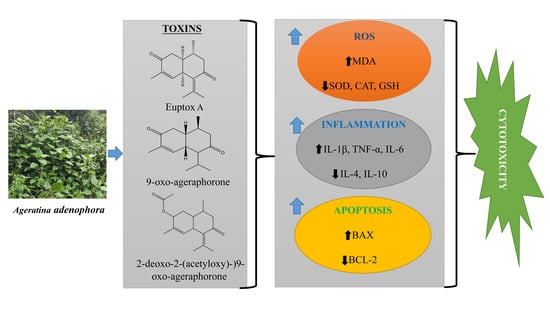
4. Molecular Mechanism of A. adenophora Toxicity
5. Pharmacological Applications of A. adenophora and Potential Therapeutic Interventions against Its Toxicity
5.1. Anti-Oxidant Therapeutic Candidates for A. adenophora Toxicity
5.2. Anti-Inflammatory Therapeutic Candidates for A. adenophora Toxicity
5.3. Degrading Microbes and Probiotics Therapeutic Candidates for A. adenophora Toxicity
6. Discussion and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nie, X.; Lv, S.; Zhang, Y.; Du, X.; Wang, L.; Biradar, S.S.; Tan, X.; Wan, F.; Weining, S. Complete Chloroplast Genome Sequence of a Major Invasive Species, Crofton Weed (Ageratina adenophora). PLoS ONE 2012, 7, e36869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.M.; Liu, J.H.; Cheng, X.; Zhang, G.F.; Fang, Y.M.; Zhang, H.J. Effects of Ageratina adenophora on Spore Germination and Gametophyte Development of Neocheiropteris palmatopedata. Am. Fern J. 2012, 102, 208–215. [Google Scholar] [CrossRef]
- Sun, W.; Zeng, C.; Yue, D.; Liu, S.; Ren, Z.; Zuo, Z.; Deng, J.; Peng, G.; Hu, Y. Ageratina adenophora causes spleen toxicity by inducing oxidative stress and pyroptosis in mice. R. Soc. Open Sci. 2019, 6, 190127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, D.; Mal, G.; Kannan, A.; Bhar, R.; Sharma, R.; Singh, B. Degradation of euptox A by tannase-producing rumen bacteria from migratory goats. J. Appl. Microbiol. 2017, 123, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.M. Investigations into Crofton weed (Eupatorium adenophorum) toxicity in horses. Aust. Vet. J. 1985, 62, 30–32. [Google Scholar] [CrossRef]
- Verma, A.; Yadava, B.P.S.; Sampath, K.T. Possible Use of Eupatorium adenophorum Spreng. in Animal Feeding. Indian J. Anim. Nutr. 1987, 4, 189–192. [Google Scholar]
- Oelrichs, P.B.; Calanasan, C.A.; Macleod, J.K.; Seawright, A.A.; Ng, J.C. Isolation of a compound from Eupatorium adenophorum (Spreng.) [Ageratina adenophora (Spreng.)] causing hepatotoxicity in mice. Nat. Toxins 1995, 3, 350–354. [Google Scholar] [CrossRef]
- Kaushal, V.; Dawra, R.K.; Sharma, O.P.; Kurade, N.P. Biochemical Alterations in the Blood Plasma of Rats Associated with Hepatotoxicity Induced by Eupatorium adenophorum. Vet. Res. Commun. 2001, 25, 601–608. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, W.; Hu, Y.; Luo, B.; Wu, L.; Qiao, Y.; Mo, Q.; Xu, R.; Zhou, Y.; Ren, Z.; et al. E. adenophorum Induces Cell Cycle and Apoptosis of Renal Cells through Mitochondrial Pathway and Caspase Activation in Saanen Goat. PLoS ONE 2015, 10, e0138504. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Mo, Q.; Hu, Y.; Chen, W.; Luo, B.; Wu, L.; Qiao, Y.; Xu, R.; Zhou, Y.; Zuo, Z.; et al. E. adenophorum induces Cell Cycle Arrest and Apoptosis of Splenocytes through the Mitochondrial Pathway and Caspase Activation in Saanen Goats. Sci. Rep. 2015, 5, 15967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Mo, Q.; Hu, Y.; Luo, B.; Wei, Y. Induction of Apoptosis and Autophagy via Mitochondria- and PI3K/Akt/mTOR-mediated Pathways by E. adenophorum in Hepatocytes of Saanen Goat. Oncotarget 2016, 7, 54537–54548. [Google Scholar] [CrossRef] [Green Version]
- Singh, Y.D.; Mukhopadhayay, S.K.; Shah, M.A.A.; Ali, M.A.; Tolenkhomba, T.C. Effects of Eupatorium ade-nophorum on Antioxidant Enzyme Status in a Mice Model. Int. J. Pharm. Pharm. Sci. 2012, 4, 436–439. [Google Scholar]
- Ouyang, C.-B.; Liu, X.-M.; Yan, D.-D.; Li, Y.; Wang, Q.-X.; Cao, A.-C.; Guo, M.-X. Immunotoxicity assessment of cadinene sesquiterpenes from Eupatorium adenophorum in mice. J. Integr. Agric. 2016, 15, 2319–2325. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Zeng, C.-R.; Yue, D.; Hu, Y.-C. Involvement of mitochondrial dysfunction in hepatotoxicity induced by Ageratina adenophora in mice. J. Zhejiang Univ. Sci. B 2019, 20, 693–698. [Google Scholar] [CrossRef]
- Sang, W.; Zhu, L.; Axmacher, J. Invasion pattern of Eupatorium adenophorum Spreng in southern China. Biol. Invasions 2009, 12, 1721–1730. [Google Scholar] [CrossRef]
- Wang, C.; Lin, H.; Feng, Q.; Jin, C.; Cao, A.; He, L. A New Strategy for the Prevention and Control of Eupatorium adenophorum under Climate Change in China. Sustainability 2017, 9, 2037. [Google Scholar] [CrossRef] [Green Version]
- Hui, L.; Sheng, Q.; Yaling, Q. Physiological Response of Different Croftonweed (Eupatorium adenophorum) Popula-tions to Low Temperature. Weed Sci. 2008, 2, 196–202. [Google Scholar]
- Sun, W.; Zeng, C.; Liu, S.; Fu, J.; Hu, L.; Shi, Z.; Yue, D.; Ren, Z.; Zhong, Z.; Zuo, Z.; et al. Ageratina adenophora induces mice hepatotoxicity via ROS-NLRP3-mediated pyroptosis. Sci. Rep. 2018, 8, 16032. [Google Scholar] [CrossRef] [Green Version]
- Auld, B.A. The distribution of Eupatorium adenophorum Spreng. on the far north coast of New South Wales. J. Proc. R. Soc. N. S. W. 1969, 102, 159–161. [Google Scholar]
- Lu, P.; Sang, W.G.; Ma, K.P. Progress and prospects in research of an exotic invasive species, Eupatorium adenophorum. Acta Phytoecol. Sin. 2005, 29, 1029–1037. [Google Scholar]
- Wang, C.; Liu, W.; Liu, L.; Cui, J. Plant diversity of different replaced communities after Eupatorium adenophorum removal. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2006, 17, 377–383. [Google Scholar]
- Tripathi, Y.; Saini, N.; Anjum, N.; Verma, P. A review of Ethnomedicinal, Phytochemical, Pharmacological and Toxicological Aspects of Eupatorium adenophorum Spreng. Asian J. Biomed. Pharm. Sci. 2018, 8, 25–35. [Google Scholar]
- Wolff, M.A. Winning the War of Weeds: The Essential Gardener’s Guide to Weed Identification and Control; Kangaroo Press: Ken-thurst, NSW, Australia, 1999; p. 17. [Google Scholar]
- Scher, J. Federal Noxious Weed Disseminules of the U.S.: An Interactive Identification Tool for Seeds and Fruit of Plants on the United States Federal Noxious Weed List, CDROM; Animal and Plant Health Inspection Service (APHIS), United States Department of Agriculture (USDA): CA, USA, 2005. [Google Scholar]
- Cronk, Q.C.B.; Fuller, J.L. Plant Invaders: The Threat to Natural Ecosystems; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Zárybnický, T.; Boušová, I.; Ambrož, M.; Skálová, L. Hepatotoxicity of Monoterpenes and Sesquiterpenes. Arch. Toxicol. 2018, 92, 1–13. [Google Scholar] [CrossRef]
- Ouyang, C.; Liu, X.; Liu, Q.; Bai, J.; Li, H.; Li, Y.; Wang, Q.; Yan, D.; Mao, L.; Cao, A.; et al. Toxicity Assessment of Cadinene Sesquiterpenes from Eupatorium adenophorum in Mice. Nat. Prod. Bioprospect. 2014, 5, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Cao, A.; Guo, M.; Liu, X.; Liu, X.; Liang, H.; Zhou, B. Identification of 9-oxo-10,11-dehydroagerophorone in Eupatorium adenophorum by HPLC. Chin. Bull. Bot. 2011, 46, 470–475. [Google Scholar]
- Okyere, S.K.; Wen, J.; Cui, Y.; Xie, L.; Gao, P.; Wang, J.; Wang, S.; Hu, Y. Toxic mechanisms and pharmacological properties of euptox A, a toxic monomer from A. adenophora. Fitoteapia 2021, 155, 105032. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Singh, A.; Sharma, O.P.; Dawra, R.K.; Kurade, N.P.; Mahato, S.B. Hepatotoxicity and Cholestasis in Rats Induced by the Sesquiterpene, 9-oxo-10,11-dehydroageraphorone, Isolated from Eupatorium adenophorum. J. Biochem. Mol. Toxicol. 2001, 15, 279–286. [Google Scholar] [CrossRef]
- Mo, Q.; Hu, L.; Weng, J.; Zhang, Y.; Zhou, Y.; Xu, R.; Zuo, Z.; Deng, J.; Ren, Z.; Zhong, Z.; et al. Euptox A induces G1 Arrest and Autophagy via P38 MAPK- and PI3K/Akt/mTOR-mediated Pathways in Mouse Splenocytes. J. Histochem. Cytochem. 2017, 65, 543–558. [Google Scholar] [CrossRef] [Green Version]
- Okyere, S.K.; Mo, Q.; Pei, G.; Ren, Z.; Deng, J.; Hu, Y. Euptox A Induces G0/GI Arrest and Apoptosis of Hepato-cyte via ROS, Mitochondrial Dysfunction and Caspases-dependent Pathways in vivo. J. Toxicol. Sci. 2020, 45, 661–671. [Google Scholar] [CrossRef]
- Ren, Z.; Gao, P.; Okyere, S.; Cui, Y.; Wen, J.; Jing, B.; Deng, J.; Hu, Y. Ageratina adenophora Inhibits Spleen Immune Function in Rats via the Loss of the FRC Network and Th1–Th2 Cell Ratio Elevation. Toxins 2021, 13, 309. [Google Scholar] [CrossRef]
- Hosseini, M.J.; Shaki, F.; Ghazi-Khansari, M.; Pourahmad, J. Toxicity of Arsenic (III) on Isolated Liver Mitochondria: A New Mechanistic Approach. Iran. J. Pharm. Res. 2013, 12, 119–136. [Google Scholar]
- Fu, J.; Hu, Y.; Chen, W.; Weng, J.; Hu, L.; Wen, S. Dosage-dependent Effects of Eupatorium adenophorum on Saanen Goat Blood Levels and the Histopathology of Several Organs. Pratacult. Sci. 2018, 35, 434–440. [Google Scholar]
- Irazabal, M.V.; Torres, V.E. Reactive Oxygen Species and Redox Signaling in Chronic Kidney Disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Chelombitko, M.A. Role of Reactive Oxygen Species in Inflammation: A Minireview. Mosc. Univ. Biol. Sci. Bull. 2018, 73, 199–202. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Lu, G.; Xu, Y.; Luo, D.; Ren, Q.; Wu, S.; Sun, C. Melatonin Alleviates Inflammasome-Induced Pyroptosis Through Inhibiting NF-κB/GSDMD Signal in Mice Adipose Tissue. J. Pineal Res. 2017, 29, e12414. [Google Scholar] [CrossRef]
- Jorgensen, I.; Rayamajhi, M.; Miao, E.A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 2017, 17, 151–164. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, X.; Jiang, W.; Li, W.; Zhu, S.; Liao, C.; Zou, L.; Ding, R.; Chen, J. Advanced Oxidation Protein Products Induce Micro-glia-mediated Neuroinflammation via MAPKs-NF-κB Signaling Pathway and Pyroptosis after Secondary Spinal Cord Injury. J. Neuroinflamm. 2020, 17, 90. [Google Scholar] [CrossRef]
- Boucher, D.; Chan, A.; Ross, C.; Schroder, K. Quantifying Caspase-1 Activity in Murine Macrophages. Clin. Appl. Mass Spectrom. Biomol. Anal. 2018, 1725, 163–176. [Google Scholar] [CrossRef]
- Kuriakose, T.; Kanneganti, T.-D. Pyroptosis in Antiviral Immunity. Curr. Top. Microbiol. Immunol. 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Qian, Z.; Zhao, Y.; Wan, C.; Deng, Y.; Zhuang, Y.; Xu, Y.; Zhu, Y.; Lu, S.; Bao, Z. Pyroptosis in the Initiation and Progression of Atherosclerosis. Front. Pharmacol. 2021, 12, 652963. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal. Transduct. Target. Ther. 2021, 6, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cheng, A.; Cheng, K.; Deng, Q.; Zhang, S.; Lan, Z.; Wang, W.; Chen, J. New Insights into the Mechanisms of Pyroptosis and Implications for Diabetic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 7057. [Google Scholar] [CrossRef]
- Lu, F.; Lan, Z.; Xin, Z.; He, C.; Guo, Z.; Xia, X.; Hu, T. Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J. Cell. Physiol. 2020, 235, 3207–3221. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Okyere, S.K.; Gao, P.; Wen, J.; Cao, S.; Wang, Y.; Deng, J.; Hu, Y. Ageratina adenophora Disrupts the Intestinal Structure and Immune Barrier Integrity in Rats. Toxins 2021, 13, 651. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, V.; Sisodia, R.; Walia, S.; Sati, O.P.; Kular, J.; Kundu, A. Chemical Analysis of Essential Oils of Eupatorium adenophorum and Their Antimicrobial, Antioxidant and Phytotoxic Properties. J. Pest. Sci. 2014, 87, 341–349. [Google Scholar] [CrossRef]
- Sharma, O.P.; Dawra, R.K.; Kurade, N.P.; Sharma, P.D. A Review of the Toxicosis and Biological Properties of the genus Eupatorium. Nat. Toxins 1998, 6, 1–14. [Google Scholar] [CrossRef]
- Awah, F.M.; Uzoegwu, P.N.; Ifeonu, P.; Oyugi, J.O.; Rutherford, J.; Yao, X.; Fehrmann, F.; Fowke, K.; Eze, M.O. Free radical scavenging activity, phenolic contents and cytotoxicity of selected Nigerian medicinal plants. Food Chem. 2012, 131, 1279–1286. [Google Scholar] [CrossRef]
- Fu, J.; Hu, L.; Shi, Z.; Wei, S.; Dong, Y.; Ya, W.; Ma, X.; Ren, Z.; Zuo, Z.; Peng, G.; et al. Two metabolites isolated from endophytic fungus Coni-ochaeta sp. F-8 in Ageratina adenophora exhibit antioxidative activity and cytotoxicity. Nat. Prod. Res. 2019, 35, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Weng, J.; Hu, L.; Peng, G.; Ren, Z.; Deng, J.; Jia, Y.; Wang, C.; He, H.; Hu, Y. Anti-NDV activity of 9-oxo10,11-dehydroageraphorone extracted from Eupatorium adenophorum Spreng in vitro. Nat. Prod. Res. 2017, 32, 2244–2247. [Google Scholar] [CrossRef] [PubMed]
- Nong, X.; Ren, Y.-J.; Wang, J.-H.; Xie, Y.; Fang, C.-L.; Yang, D.-Y.; Liu, T.-F.; Zhang, R.-H.; Chen, L.; Gu, X.-B.; et al. Clinical efficacy of botanical extracts from Eupatorium adenophorum against the Sarcoptes scabiei (Sarcoptidae: Sarcoptes) in rabbits. Vet. Parasitol. 2013, 195, 157–164. [Google Scholar] [CrossRef]
- Rajeswary, M.; Govindarajan, M.; Murugan, K.; Hwang, J.S.; Barnard, D.R.; Amsath, A.; Muthukumaran, U. Ovicidal Efficacy Of Ageratina adenophora (Family:Asteraceae) against Anopheles Stephensi (Diptera: Culicidae). Int. J. Pure Appl. Zool. 2014, 2, 196–199. [Google Scholar]
- Das, R. Antifungal activities and phytochemical screening of two invasive alien species of Nepal. Stud. Fungi 2018, 3, 293–301. [Google Scholar] [CrossRef]
- Pandey, A.K.; Mohan, M.; Singh, P.; Palni, U.T.; Tripathi, N. Chemical composition, antibacterial and antioxidant activity of essential oil of Eupatorium adenophorum Spreng. from Eastern Uttar Pradesh, India. Food Biosci. 2014, 7, 80–87. [Google Scholar] [CrossRef]
- Arvind, N.; Amit, S. Antimicrobial Potential of Eupatorium adenophorum Spreng. Phcog. J. 2010, 2, 61–64. [Google Scholar]
- Ramya, N.; Mayuri, P.K.; Santny, A.S.; Angayarkanni, T. Antimicrobial efficacy of silver nanoparticles conju-gated with Ageratina adenophora leaf extract. Asia Pac. J. Res. 2015, 1, 170–176. [Google Scholar]
- Liu, X.; Ouyang, C.; Li, Y.; Yang, D.; Fang, W.; Yan, D.; Guo, M.; Cao, A.; Wang, Q. Evaluation of the antimicrobial activity of 9-oxo-agerophorone against soil borne pathogens. J. Plant Dis. Prot. 2016, 123, 163–170. [Google Scholar] [CrossRef]
- Dong, L.-M.; Zhang, M.; Xu, Q.-L.; Zhang, Q.; Luo, B.; Luo, Q.-W.; Liu, W.-B.; Tan, J.-W. Two New Thymol Derivatives from the Roots of Ageratina adenophora. Molecules 2017, 22, 592. [Google Scholar] [CrossRef] [Green Version]
- Kundu, A.; Saha, S.; Walia, S.; Shakil, N.A.; Kumar, J.; Annapurna, K. Cadinene sesquiterpenes from Eupatorium adenophorum and their antifungal activity. J. Environ. Health B 2013, 48, 516–522. [Google Scholar] [CrossRef]
- Zheng, G.; Luo, S.; Li, S.; Hua, J.; Li, W.; Li, S. Specialized metabolites from Ageratina adenophora and their inhibitory activities against pathogenic fungi. Phytochemistry 2018, 148, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, Y.; Liu, R.; Xie, X. Study on the inhibitory effect of Eupatorium adenophorum extract on two plant pathogenic fungi. Hubei Agric. Sci. 2012, 51, 1133–1135. [Google Scholar]
- Liu, B.; Cao, L.; Zhang, L.; Yuan, X.; Zhao, B. Preparation, Phytochemical Investigation, and Safety Evaluation of Chlorogenic Acid Products from Eupatorium adenophorum. Molecules 2016, 22, 67. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ouyang, C.; Wang, Q.; Li, Y.; Yan, D.; Yang, D.; Guo, M.; Cao, A. Evaluation of antibacterial and anti-fungal properties of 9-oxo-10,11-dehydroageraphorone extracted from Eupatorium adenophorum. J. Plant Dis. Prot. 2016, 123, 93–99. [Google Scholar] [CrossRef]
- Hu, L.; Hu, Y.; Zhang, Y.; Shi, Z.; Fu, J.; Sun, W.; Deng, J.; Ren, Z.; Zuo, Z.; Cao, S.; et al. Inhibition effect of Ageratina adenophora is methanol extracts against Microsporidium gypsum and its mechanism. Acta Agric. Zhejiangensis 2019, 31, 1555–1562. [Google Scholar]
- Liu, X.; Ouyang, C.; Wang, Q.; Li, Y.; Yan, D.; Yang, D.; Fang, W.; Cao, A.; Guo, M. Effects of oil extracts of Eupatorium adenophorum on Phytophthora capsici and other plant pathogenic fungi in vitro. Pestic Biochem. Physiol. 2017, 140, 90–96. [Google Scholar] [CrossRef]
- Lin, Y.; He, S.-Q.; Lu, Z.-H.; Gao, Y.-L.; Duan, Y.-R.; Li, Z.-Y.; Chen, B.; Gui, F.-R. Influence of Aphis gossypii feeding on defense strategy of native and introduced populations of Ageratina adenophora. Arthropod-Plant Interact. 2020, 14, 345–356. [Google Scholar] [CrossRef]
- Xu, R.; Wu, D.; Zhang, W.D.; Yin, F.; Kuang, R.P. Efficacy of Ageratina adenophora extract and biogas fermentation residue against the cabbage aphid, Brevicoryne brassicae and an assessment of the risk to the parasi-toid Diaeretiella rapae. Int. J. Pest Manag. 2009, 55, 151–156. [Google Scholar] [CrossRef]
- Wang, Y. The toxicities of the extracts from Eupatorium adenophorum against Aphis gossypii and their aphid-killing mechanism. J. Plant Prot. 2002, 29, 337–340. [Google Scholar]
- André, R.; Catarro, J.; Freitas, D.; Pacheco, R.; Oliveira, M.C.; Serralheiro, M.L.; Falé, P.L. Action of Euptox A from Ageratina adenophora Juice on Human Cell Lines: A Top-down Study Using FTIR Spectroscopy and Protein Profiling. Toxicol. Vitr. 2019, 57, 217–225. [Google Scholar] [CrossRef]
- Liao, F.; Wang, Y.; Huang, Y.; Mo, Q.; Tan, H.; Wei, Y.; Hu, Y. Isolation and identification of bacteria capable of degrading euptox A from Eupatorium adenophorum Spreng. Toxicon 2014, 77, 87–92. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, B.; Yang, J.; Ma, X.; Deng, S.; Huang, Y.; Wen, Y.; Yuan, J.; Yang, X. Essential Oil Derived from Eupatorium adenophorum Spreng. Mediates Anticancer Effect by Inhibiting STAT3 and AKT Activation to Induce Apoptosis in Hepatocellular Carcinoma. Front. Pharmacol. 2018, 9, 483. [Google Scholar] [CrossRef]
- Ringmichon, C.L.; Gopalkrishnan, B. Antipyretic activity of Eupatorium adenophorum leaves. Int. J. Appl. Biol. Pharm. 2017, 8, 1–4. [Google Scholar]
- Mandal, P.; Sinha Babu, S.P.; Mandal, N.C. Antimicrobial Activity of Saponins from Acacia auriculiformis. Fitoterapia 2005, 76, 462–465. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.; Sharma, D.K.; Kishore, K. Evaluation of wound healing activity of Ageratina adenophora (Spreng.) R. M. King & H. Rob. Int. J. Pharma Res. Health Sci. 2017, 5, 1873–1876. [Google Scholar]
- Tiwary, B.K.; Bihani, S.; Kumar, A.; Chakraborty, R.; Ghosh, R. The In Vitro Cytotoxic Activity of Eth-no-pharmacological Important Plants of Darjeeling District of West Bengal against Different Human Cancer Cell Lines. BMC Complement. Altern. Med. 2015, 15, 22. [Google Scholar] [CrossRef] [Green Version]
- Palá-Paúl, J.; Pérez-Alonso, M.; Velasco-Negueruela, A.; Sanz, J. Analysis by gas chromatography–mass spectrometry of the volatile components of Ageratina adenophora Spreng.; growing in the Canary Islands. J. Chromatogr. A 2002, 947, 327–331. [Google Scholar] [CrossRef]
- Khazeo, P.; Mazumder, M.U.; Puro, K.N.; Jyrwa, R.; Jamir, N.; Sailo, L. In vitro Antioxidant Activity of Methanolic Extracts of Ageratum conyzoides and Ageratina adenophora Leaves. Perspect. Trends Dev. Sci. Educ. Res. 2018, 178, 169–172. [Google Scholar]
- Amevor, F.K.; Cui, Z.; Du, X.; Ning, Z.; Shu, G.; Jin, N.; Deng, X.; Tian, Y.; Zhang, Z.; Kang, X.; et al. Combination of Quercetin and Vitamin E Supple-mentation Promotes Yolk Precursor Synthesis and Follicle Development in Aging Breeder Hens via Liver-Blood-Ovary Signal Axis. Animals 2021, 11, 1915. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 activator, Ameliorates Aging-related Progressive Renal Injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Yang, F.; Meng, L.; Chen, D.; Wang, M.; Lu, X.; Chen, D.; Jiang, Y.; Xing, N. Lycopene attenuates chronic prostatitis/chronic pelvic pain syndrome by inhibiting oxidative stress and inflammation via the interaction of NF-κB, MAPKs, and Nrf2 signaling pathways in rats. Andrology 2020, 8, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Zeng, C.; Okyere, S.K.; Chen, Z.; Hu, Y. Glycine nano-selenium prevents brain oxidative stress and neurobehavioral abnormalities caused by MPTP in rats. J. Trace Elements Med. Biol. 2021, 64, 126680. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, B.; Sun, X.; Li, Z.; Chen, Y.; Guo, Z.; Liu, H.; Li, D.; Wang, C.; Zhu, X.; et al. Protective effects of alfalfa saponins on oxidative stress-induced apoptotic cells. Food Funct. 2020, 11, 8133–8140. [Google Scholar] [CrossRef]
- Li, F.; Xiang, H.; Lu, J.; Chen, Z.; Huang, C.; Yuan, X. Lycopene ameliorates PTSD-like behaviors in mice and rebalances the neuroinflammatory response and oxidative stress in the brain. Physiol. Behav. 2020, 224, 113026. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zou, Y.; Peng, J. Oregano Essential Oil Attenuates RAW264.7 Cells from Lipopolysaccharide-Induced Inflammatory Response through Regulating NADPH Oxidase Activation-Driven Oxidative Stress. Molecules 2018, 23, 1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Feng, X.; Zheng, D.; Li, A.; Li, C.; Li, S.; Zhao, Z. Ergosterol attenuates cigarette smoke extract-induced COPD by modulating inflammation, oxidative stress and apoptosis in vitro and in vivo. Clin. Sci. 2019, 133, 1523–1536. [Google Scholar] [CrossRef]
- Kulkarni, R.A.; Deshpande, A.R. Anti-inflammatory and antioxidant effect of ginger in tuberculosis. J. Complement. Integr. Med. 2016, 13, 201–206. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.; Wang, Z.; Lin, X.; Tian, Y.; Zhao, Q.; Zheng, P. Anti-inflammatory effect of selenium on lead-induced testicular inflammation by inhibiting NLRP3 inflammasome activation in chickens. Theriogenology 2020, 155, 139–149. [Google Scholar] [CrossRef]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macro-phages Stimulated with LPS. Evid. Based Complement. Altern. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef] [PubMed]
- Poudel, R.; Neupane, N.P.; Mukeri, I.H.; Alok, S.; Verma, A. An updated review on invasive nature, phyto-chemical evaluation, & pharmacological activity of Ageratina adenophora. Int. J. Pharm. Sci. Res. 2020, 11, 2510–2520. [Google Scholar]
- Chakravarty, A.K.; Mazumder, T.; Chatterjee, S.N. Anti-Inflammatory Potential of Ethanolic Leaf Extract of Eupatorium adenophorum Spreng. Through Alteration in Production of TNF-α, ROS and Expression of Certain Genes. Evid. Based Complement. Altern. Med. 2011, 2011, 471074. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Mal, G.; Marotta, F. Designer Probiotics: Paving the Way to Living Therapeutics. Trends Biotechnol. 2017, 35, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Chettri, B.; Singha, N.A.; Mukherjee, A.; Rai, A.N.; Chattopadhyay, D.; Singh, A.K. Hydrocarbon Degradation Potential and Competitive Persistence of Hydrocarbonoclastic Bacterium Acinetobacter pittii strain ABC. Arch. Microbiol. 2019, 201, 1129–1140. [Google Scholar] [CrossRef]
- Hashmat, A.J.; Afzal, M.; Fatima, K.; Anwar-Ul-Haq, M.; Khan, Q.M.; Arias, C.A.; Brix, H. Characterization of Hydrocarbon-Degrading Bacteria in Constructed Wetland Microcosms Used to Treat Crude Oil Polluted Water. Bull. Environ. Contam. Toxicol. 2019, 102, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Risa, A.; Krifaton, C.; Kukolya, J.; Kriszt, B.; Cserháti, M.; Táncsics, A. Aflatoxin B1 and Zearalenone-Detoxifying Profile of Rhodococcus Type Strains. Curr. Microbiol. 2018, 75, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Han, K.J.; Lee, J.E.; Lee, N.K.; Paik, H.D. Antioxidant and Anti-Inflammatory Effect of Probiotic Lactobacillus plantarum KU15149 Derived from Korean Homemade Diced-Radish Kimchi. J. Microbiol. Biotechnol. 2020, 30, 591–598. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.; Baptista, A.A.S.; Valdiviezo, M.J.J.; Justino, L.; Menck-Costa, M.F.; Ferraz, C.R.; da Gloria, E.M.; Verri, W.A., Jr.; Bracarense, A.P.F. Lactobacillus spp. reduces morphological changes and oxidative stress induced by deoxynivalenol on the intestine and liver of broilers. Toxicon 2020, 185, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Park, D.; Kim, Y.J.; Lee, I.; Kim, S.; Oh, C.; Kim, J.; Yang, J.; Jo, S. Lactobacillus salivarius BP121 prevents cisplatin-induced acute kidney injury by inhibition of uremic toxins such as indoxyl sulfate and p-cresol sulfate via alleviating dysbiosis. Int. J. Mol. Med. 2020, 45, 1130–1140. [Google Scholar] [CrossRef] [Green Version]
- Fernández, J.; Silván, B.; Entrialgo-Cadierno, R.; Villar, C.J.; Capasso, R.; Uranga, J.A.; Lombó, F.; Abalo, R. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed. Pharmacother. 2021, 143, 112241. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; Del Castillo, M.D.; Abalo, R. Cannabidiol and Other Non-Psychoactive Cannabinoids for Prevention and Treatment of Gastrointestinal Disorders: Useful Nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067. [Google Scholar] [CrossRef]
- Freitas, M.; Vasconcelos, A.; Gonçalves, E.; Ferrarini, E.; Vieira, G.; Cicia, D.; Cola, M.; Capasso, R.; Dutra, R. Involvement of Opioid System and TRPM8/TRPA1 Channels in the Antinociceptive Effect of Spirulina platensis. Biomolecules 2021, 11, 592. [Google Scholar] [CrossRef]


| Antioxidant Agents | Animal Model | Dosage | Activities | Reference | |
|---|---|---|---|---|---|
| 1 | Quercetin and vitamin E combination | Chicken | 0.4 g/kg and 0.2 g/kg respectively for 10 weeks | Reduce ROS Increase total antioxidant capacity (T-AOC) Reduce pro-inflammation cytokines | [80] |
| 2 | Resveratrol | Mice | 40 mg/kg for 6 months | Reduce ROS Reduce pro-inflammation cytokines | [81] |
| 3 | Lycopene | Rat | 10 and 20 mg/kg for 30 days | Reduce ROS Reduce pro-inflammation cytokines (IL-6, IL-1β, TNF-α) | [82] |
| 4 | Glycine Nano-selenium | Rats | 0.05 and 0.1 mg/kg for 30 days | Decrease the MDA levels | [83] |
| 5 | Alfalfa saponins | IEC-6 cells | 75, 100, 150, 200 and 300 μmol/L for 24 h | Elevate the amount of T-AOC in cells | [84] |
| 6 | Malus doumeri leaf flavonoids | human embryonic kidney 293 T cells | 160 μg/mL for 48 h | Increase the levels of catalase (CAT), superoxide dismutase (SOD), glutathione (GSH), and glutathione peroxidase (GSH-Px) and reduce the level of malondialdehyde (MDA) | [85] |
| 7 | Oregano essential oil | RAW264.7 Cells | 2.5–10 μg/mL for 24 h | Inhibited the mRNA expression of IL-1β, IL-6, and TNF-α in the RAW264.7 cells | [86] |
| 8 | Ergosterol | 16 HBE cells and Balb/c mice | 5, 10 and 20 μM for 24 h and 40 mg/kg for 21 days | Decrease the expression of interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), | [87] |
| 9 | Ginger | Pulmonary TB patients (human) | 3 g of ginger extract daily for 1 month | Reduced the levels of tumor necrosis factor (TNF) alpha | [88] |
| 10 | Selenium | Chicken | 1 mg/kg for 12 weeks | Reduced the levels of inflammation-related factors (Nuclear factor-kappa B, tumor necrosis factor-α, cyclooxygenase-2, NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain, caspase-1, interleukin (IL)-1β, IL-6, IL-18 and interferon-γ) | [89] |
| 11 | Probiotics (Lactobacillus acidophilus, Lactobacillus casei, Lactococcus lactis, Lactobacillus reuteri, and Saccharomyces boulardii) | Human colon epithelial HT-29 cells | 108 CFU/mL for 18 h | Reduce IL-1β, IL-6, TNF-α, and increase IL-10 production Increased % of DPPH scavenging activity | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Okyere, S.K.; Wen, J.; Xie, L.; Cui, Y.; Wang, S.; Wang, J.; Cao, S.; Shen, L.; Ma, X.; et al. An Overview: The Toxicity of Ageratina adenophora on Animals and Its Possible Interventions. Int. J. Mol. Sci. 2021, 22, 11581. https://doi.org/10.3390/ijms222111581
Ren Z, Okyere SK, Wen J, Xie L, Cui Y, Wang S, Wang J, Cao S, Shen L, Ma X, et al. An Overview: The Toxicity of Ageratina adenophora on Animals and Its Possible Interventions. International Journal of Molecular Sciences. 2021; 22(21):11581. https://doi.org/10.3390/ijms222111581
Chicago/Turabian StyleRen, Zhihua, Samuel Kumi Okyere, Juan Wen, Lei Xie, Yujing Cui, Shu Wang, Jianchen Wang, Suizhong Cao, Liuhong Shen, Xiaoping Ma, and et al. 2021. "An Overview: The Toxicity of Ageratina adenophora on Animals and Its Possible Interventions" International Journal of Molecular Sciences 22, no. 21: 11581. https://doi.org/10.3390/ijms222111581