Interactions of the Lysosomotropic Detergent O-Methyl-Serine Dodecylamide Hydrochloride (MSDH) with Lipid Bilayer Membranes—Implications for Cell Toxicity
Abstract
:1. Introduction
2. Results
2.1. MSDH-induced Leakage of Small and Large Molecules is Affected by pH and Lipid Composition
2.2. MSDH Causes Permeabilization of Cellular Membranes and Cell Death
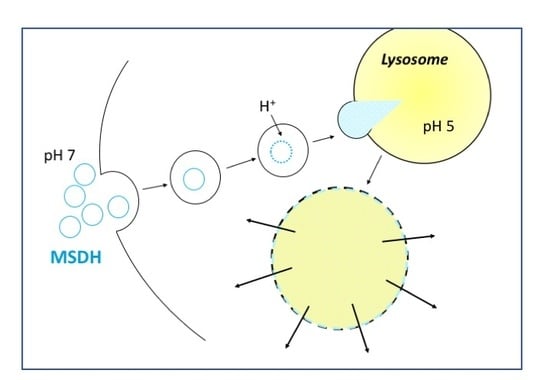
2.3. Uptake of MSDH by Endocytosis
3. Discussion
4. Materials and Methods
4.1. MSDH
4.2. Preparation of Liposomes
4.3. Fluorophore Leakage Experiments
4.4. FCS Measurements
4.5. Cells and Culture Conditions
4.6. Viability and Apoptosis Analysis
4.7. Immunocytochemistry
4.8. Cytosolic Extraction
4.9. Determination of β-N-acetyl-glucosaminidase (NAG) Activity
4.10. Western Blot
4.11. TEM
4.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANTS | 8-Aminonapthalene-1,3,6 trisulfonic acid |
| DPX | p-Xylene-bis-pyridinium bromide |
| FCS | Fluorescence correlation spectroscopy |
| LAMP2 | Lysosomal-associated membrane protein-2 |
| LD | Lysosomotropic detergent |
| LDH | Lactate dehydrogenase |
| LLOMe | L-leucyl-L-leucine methyl ester |
| LMP | Lysosomal membrane permeabilization |
| MDC | Mono-dansylcadaverine |
| MSDH | O-methyl-serine dodecylamine hydrochloride |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NAG | β-N-acetyl-glucosaminidase |
| TRITC-dextran 40 | Tetra-methyl rhodamine isothiocyanate–dextran with MW 40 kDa |
References
- Appelqvist, H.; Waster, P.; Kagedal, K.; Ollinger, K. The Lysosome: From Waste Bag to Potential Therapeutic Target. J. Mol. Cell Biol. 2013, 5, 214–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, A.R.A.; Saftig, P. Lysosomal Storage Disorders—Challenges, Concepts and Avenues for Therapy: Beyond Rare Diseases. J. Cell Sci. 2019, 132, 2. [Google Scholar] [CrossRef] [PubMed]
- Kagedal, K.; Zhao, M.; Svensson, I.; Brunk, U.T. Sphingosine-Induced Apoptosis Is Dependent on Lysosomal Proteases. Biochem. J. 2001, 359, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yuan, X.; Nordgren, G.; Dalen, H.; Dubowchik, G.M.; Firestone, R.A.; Brunk, U.T. Induction of Cell Death by the Lysosomotropic Detergent MSDH. FEBS Lett. 2000, 470, 35–39. [Google Scholar] [CrossRef]
- Serrano-Puebla, A.; Boya, P. Lysosomal Membrane Permeabilization in Cell Death: New Evidence and Implications for Health and Disease. Ann. N. Y. Acad. Sci. 2016, 1371, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Boya, P. Lysosomal Function and Dysfunction: Mechanism and Disease. Antioxid. Redox Signal. 2012, 17, 766–774. [Google Scholar] [CrossRef] [Green Version]
- Guicciardi, M.E.; Malhi, H.; Mott, J.L.; Gores, G.J. Apoptosis and Necrosis in the Liver. Compr. Physiol. 2013, 3, 977–1010. [Google Scholar]
- Johansson, A.C.; Appelqvist, H.; Nilsson, C.; Kagedal, K.; Roberg, K.; Ollinger, K. Regulation of Apoptosis-Associated Lysosomal Membrane Permeabilization. Apoptosis 2010, 15, 527–540. [Google Scholar] [CrossRef] [Green Version]
- Kirkegaard, T.; Jaattela, M. Lysosomal Involvement in Cell Death and Cancer. Biochim. Biophys. Acta 2009, 1793, 746–754. [Google Scholar] [CrossRef] [Green Version]
- Repnik, U.; Cesen, M.H.; Turk, B. Lysosomal Membrane Permeabilization in Cell Death: Concepts and Challenges. Mitochondrion 2014, 19, 49–57. [Google Scholar] [CrossRef]
- Boya, P.; Kroemer, G. Lysosomal Membrane Permeabilization in Cell Death. Oncogene 2008, 27, 6434–6451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhitomirsky, B.; Assaraf, Y.G. Lysosomal Sequestration of Hydrophobic Weak Base Chemotherapeutics Triggers Lysosomal Biogenesis and Lysosome-Dependent Cancer Multidrug Resistance. Oncotarget 2015, 6, 1143–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Duve, C.; de Barsy, T.; Poole, B.; Trouet, A.; Tulkens, P.; van Hoof, F. Commentary. Lysosomotropic Agents. Biochem. Pharmacol. 1974, 23, 2495–2531. [Google Scholar] [CrossRef]
- Seddon, A.M.; Curnow, P.; Booth, P.J. Membrane Proteins, Lipids and Detergents: Not Just a Soap Opera. Biochim. Biophys. Acta 2004, 1666, 105–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Ding, S.; Zhang, Z.; Wang, L.; You, Y. Cationic Micelle: A Promising Nanocarrier for Gene Delivery with High Transfection Efficiency. J. Gene Med. 2019, 21, e3101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghassemi, S.; Hadjizadeh, A. Nano-Niosomes as Nanoscale Drug Delivery Systems: An Illustrated Review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef]
- Lichtenberg, D.; Ahyayauch, H.; Goni, F.M. The Mechanism of Detergent Solubilization of Lipid Bilayers. Biophys. J. 2013, 105, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Helenius, A.; Simons, K. Solubilization of Membranes by Detergents. Biochim. Biophys. Acta 1975, 415, 29–79. [Google Scholar] [CrossRef]
- Dennis, E.A. Micellization and Solubilization of Phospholipids by Surfactants. Adv. Colloid Interface Sci. 1986, 26, 155–175. [Google Scholar] [CrossRef]
- Ahyayauch, H.; Bennouna, M.; Alonso, A.; Goni, F.M. Detergent Effects on Membranes at Subsolubilizing Concentrations: Transmembrane Lipid Motion, Bilayer Permeabilization, and Vesicle Lysis/Reassembly Are Independent Phenomena. Langmuir 2010, 26, 7307–7313. [Google Scholar] [CrossRef]
- Kragh-Hansen, U.; le Maire, M.; Moller, J.V. The Mechanism of Detergent Solubilization of Liposomes and Protein-Containing Membranes. Biophys. J. 1998, 75, 2932–2946. [Google Scholar] [CrossRef] [Green Version]
- Lete, M.G.; Monasterio, B.G.; Collado, M.I.; Medina, M.; Sot, J.; Alonso, A.; Goni, F.M. Fast and Slow Biomembrane Solubilizing Detergents: Insights into Their Mechanism of Action. Colloids Surf. B Biointerfaces 2019, 183, 110430. [Google Scholar] [CrossRef] [PubMed]
- Firestone, R.A.; Pisano, J.M.; Bonney, R.J. Lysosomotropic Agents. 1. Synthesis and Cytotoxic Action of Lysosomotropic Detergents. J. Med. Chem. 1979, 22, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Dubowchik, G.M.; Padilla, L.; Edinger, K.; Firestone, R.A. Amines That Transport Protons across Bilayer Membranes: Synthesis, Lysosomal Neutralization, and Two-Phase pKa Values by Nmr. J. Org. Chem. 1996, 61, 4676–4684. [Google Scholar] [CrossRef]
- Appelqvist, H.; Nilsson, C.; Garner, B.; Brown, A.J.; Kagedal, K.; Ollinger, K. Attenuation of the Lysosomal Death Pathway by Lysosomal Cholesterol Accumulation. Am. J. Pathol. 2011, 178, 629–639. [Google Scholar] [CrossRef]
- Villamil Giraldo, A.M.; Fyrner, T.; Wennmalm, S.; Parikh, A.N.; Ollinger, K.; Ederth, T. Spontaneous Vesiculation and pH-Induced Disassembly of a Lysosomotropic Detergent: Impacts on Lysosomotropism and Lysosomal Delivery. Langmuir 2016, 32, 13566–13575. [Google Scholar] [CrossRef] [Green Version]
- Stefanutti, E.; Papacci, F.; Sennato, S.; Bombelli, C.; Viola, I.; Bonincontro, A.; Bordi, F.; Mancini, G.; Gigli, G.; Risuleo, G. Cationic Liposomes Formulated with DMPC and a Gemini Surfactant Traverse the Cell Membrane without Causing a Significant Bio-Damage. Biochim. Biophys. Acta 2014, 1838, 2646–2655. [Google Scholar] [CrossRef] [Green Version]
- Sandvig, K.; Kavaliauskiene, S.; Skotland, T. Clathrin-Independent Endocytosis: An Increasing Degree of Complexity. Histochem. Cell Biol. 2018, 150, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Boucrot, E. Fast and Ultrafast Endocytosis. Curr. Opin. Cell Biol. 2017, 47, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.M.; Renden, R.; von Gersdorff, H. Synaptic Vesicle Endocytosis: Fast and Slow Modes of Membrane Retrieval. Trends Neurosci. 2008, 31, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Thiele, D.L.; Lipsky, P.E. Mechanism of L-Leucyl-L-Leucine Methyl Ester-Mediated Killing of Cytotoxic Lymphocytes: Dependence on a Lysosomal Thiol Protease, Dipeptidyl Peptidase I, That Is Enriched in These Cells. Proc. Natl. Acad. Sci. USA 1990, 87, 83–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Action of Leucyl-Leucine Methyl Ester on Cytotoxic Lymphocytes Requires Uptake by a Novel Dipeptide-Specific Facilitated Transport System and Dipeptidyl Peptidase I-Mediated Conversion to Membranolytic Products. J. Exp. Med. 1990, 172, 183–194. [CrossRef] [PubMed]
- Radulovic, M.; Schink, K.O.; Wenzel, E.M.; Nahse, V.; Bongiovanni, A.; Lafont, F.; Stenmark, H. ESCRT-Mediated Lysosome Repair Precedes Lysophagy and Promotes Cell Survival. EMBO J. 2018, 37, 21. [Google Scholar] [CrossRef] [PubMed]
- Skowyra, M.L.; Schlesinger, P.H.; Naismith, T.V.; Hanson, P.I. Triggered Recruitment of ESCRT Machinery Promotes Endolysosomal Repair. Science 2018, 360, eaar5078. [Google Scholar] [CrossRef] [Green Version]
- Aits, S.; Kricker, J.; Liu, B.; Ellegaard, A.M.; Hamalisto, S.; Tvingsholm, S.; Corcelle-Termeau, E.; Hogh, S.; Farkas, T.; Jonassen, A.H.; et al. Sensitive Detection of Lysosomal Membrane Permeabilization by Lysosomal Galectin Puncta Assay. Autophagy 2015, 11, 1408–1424. [Google Scholar] [CrossRef] [Green Version]
- Dutta, D.; Donaldson, J.G. Search for Inhibitors of Endocytosis: Intended Specificity and Unintended Consequences. Cell. Logist. 2012, 2, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.H.; Rothberg, K.G.; Anderson, R.G. Mis-Assembly of Clathrin Lattices on Endosomes Reveals a Regulatory Switch for Coated Pit Formation. J. Cell Biol. 1993, 123, 1107–1117. [Google Scholar] [CrossRef]
- Naslavsky, N.; Weigert, R.; Donaldson, J.G. Characterization of a Nonclathrin Endocytic Pathway: Membrane Cargo and Lipid Requirements. Mol. Biol. Cell 2004, 15, 3542–3552. [Google Scholar] [CrossRef] [Green Version]
- Lange, Y.; Swaisgood, M.H.; Ramos, B.V.; Steck, T.L. Plasma Membranes Contain Half the Phospholipid and 90% of the Cholesterol and Sphingomyelin in Cultured Human Fibroblasts. J. Biol. Chem. 1989, 264, 3786–3793. [Google Scholar]
- Warnock, D.E.; Roberts, C.; Lutz, M.S.; Blackburn, W.A.; Young, W.W., Jr.; Baenziger, J.U. Determination of Plasma Membrane Lipid Mass and Composition in Cultured Chinese Hamster Ovary Cells Using High Gradient Magnetic Affinity Chromatography. J. Biol. Chem. 1993, 268, 10145–10153. [Google Scholar]
- Thurston, T.L.; Wandel, M.P.; von Muhlinen, N.; Foeglein, A.; Randow, F. Galectin 8 Targets Damaged Vesicles for Autophagy to Defend Cells against Bacterial Invasion. Nature 2012, 482, 414–418. [Google Scholar] [CrossRef]
- Maejima, I.; Takahashi, A.; Omori, H.; Kimura, T.; Takabatake, Y.; Saitoh, T.; Yamamoto, A.; Hamasaki, M.; Noda, T.; Isaka, Y.; et al. Autophagy Sequesters Damaged Lysosomes to Control Lysosomal Biogenesis and Kidney Injury. EMBO J. 2013, 32, 2336–2347. [Google Scholar] [CrossRef] [Green Version]
- Appelqvist, H.; Sandin, L.; Bjornstrom, K.; Saftig, P.; Garner, B.; Ollinger, K.; Kagedal, K. Sensitivity to Lysosome-Dependent Cell Death Is Directly Regulated by Lysosomal Cholesterol Content. PLoS ONE 2012, 7, e50262. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.P.; Gleeson, P.A. Macropinocytosis: An Endocytic Pathway for Internalising Large Gulps. Immunol. Cell Biol. 2011, 89, 836–843. [Google Scholar] [CrossRef]
- Chanaday, N.L.; Kavalali, E.T. Optical Detection of Three Modes of Endocytosis at Hippocampal Synapses. Elife 2018, 7, e36097. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Jang, W.Y.; Ko, Y.T. The Effect of Surface Charges on the Cellular Uptake of Liposomes Investigated by Live Cell Imaging. Pharm. Res. 2017, 34, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.I. Pharmacological Inhibition of Endocytic Pathways: Is It Specific Enough to Be Useful? Methods Mol. Biol. 2008, 440, 15–33. [Google Scholar] [PubMed]
- Buckley, C.M.; King, J.S. Drinking Problems: Mechanisms of Macropinosome Formation and Maturation. FEBS J. 2017, 284, 3778–3790. [Google Scholar] [CrossRef] [Green Version]
- Ellegaard, A.M.; Dehlendorff, C.; Vind, A.C.; Anand, A.; Cederkvist, L.; Petersen, N.H.T.; Nylandsted, J.; Stenvang, J.; Mellemgaard, A.; Osterlind, K.; et al. Repurposing Cationic Amphiphilic Antihistamines for Cancer Treatment. EBioMedicine 2016, 9, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torchilin, V.; Weissig, V. Liposomes: A Practical Approach; OUP Oxford: Oxford, UK, 2003. [Google Scholar]
- Culbertson, C.T.; Jacobson, S.C.; Ramsey, J.M. Diffusion Coefficient Measurements in Microfluidic Devices. Talanta 2002, 56, 365–373. [Google Scholar] [CrossRef]







| Detergent | Liposomes pH 5 (milliseconds) | Liposomes pH 7 (milliseconds) | ||
|---|---|---|---|---|
| DOPC/DOPE | +Cholesterol | DOPC/DOPE | +Cholesterol | |
| Triton X-100 | 0.30 ± 0.01 | 0.33 ± 0.01 | 0.28 ± 0.01 | 0.30 ± 0.01 |
| MSDH:lipid 20:1 | 0.38 ± 0.07 | 0.48 ± 0.07 | 0.34 ± 0.04 | 0.51 ± 0.02* |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villamil Giraldo, A.-M.; Eriksson, I.; Wennmalm, S.; Fyrner, T.; Ederth, T.; Öllinger, K. Interactions of the Lysosomotropic Detergent O-Methyl-Serine Dodecylamide Hydrochloride (MSDH) with Lipid Bilayer Membranes—Implications for Cell Toxicity. Int. J. Mol. Sci. 2020, 21, 3136. https://doi.org/10.3390/ijms21093136
Villamil Giraldo A-M, Eriksson I, Wennmalm S, Fyrner T, Ederth T, Öllinger K. Interactions of the Lysosomotropic Detergent O-Methyl-Serine Dodecylamide Hydrochloride (MSDH) with Lipid Bilayer Membranes—Implications for Cell Toxicity. International Journal of Molecular Sciences. 2020; 21(9):3136. https://doi.org/10.3390/ijms21093136
Chicago/Turabian StyleVillamil Giraldo, Ana-Maria, Ida Eriksson, Stefan Wennmalm, Timmy Fyrner, Thomas Ederth, and Karin Öllinger. 2020. "Interactions of the Lysosomotropic Detergent O-Methyl-Serine Dodecylamide Hydrochloride (MSDH) with Lipid Bilayer Membranes—Implications for Cell Toxicity" International Journal of Molecular Sciences 21, no. 9: 3136. https://doi.org/10.3390/ijms21093136