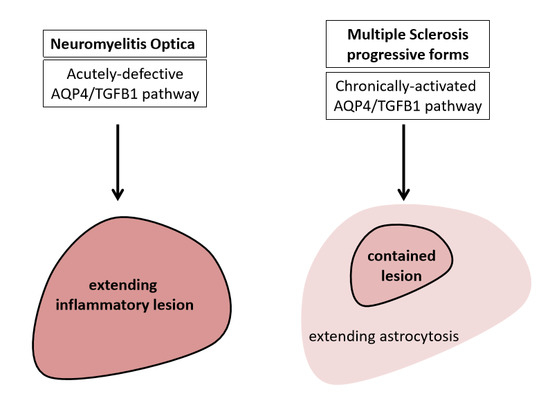
The Demonstration of an Aqp4/Tgf-Beta 1 Pathway in Murine Astrocytes Holds Implications for Both Neuromyelitis Optica and Progressive Multiple Sclerosis
Abstract
:Acknowledges
Conflicts of Interest
References
- Xue, X.; Zhang, W.; Zhu, J.; Chen, X.; Zhou, S.; Xu, Z.; Hu, G.; Su, C. Aquaporin-4 deficiency reduces TGF-β1 in mouse midbrains and exacerbates pathology in experimental Parkinson’s disease. J. Cell. Mol. Med. 2019, 23, 2568–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingerchuk, D.M.; Lennon, V.A.; Lucchinetti, C.F.; Pittock, S.J.; Weinshenker, B.G. The spectrum of neuromyelitis optica. Lancet. Neurol. 2007, 6, 805–815. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, H.-Q.; Huang, Y.; Qiu, Y.-H.; Peng, Y.-P. Transforming growth factor-β1 acts via TβR-I on microglia to protect against MPP(+)-induced dopaminergic neuronal loss. Brain Behav. Immun. 2016, 51, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.; Chang, C.-F.; Goods, B.A.; Hammond, M.D.; Mac Grory, B.; Ai, Y.; Steinschneider, A.F.; Renfroe, S.C.; Askenase, M.H.; McCullough, L.D.; et al. TGF-β1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J. Clin. Investig. 2016, 127, 280–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccarelli, O.; Cohen, J.A.; Reingold, S.C.; Weinshenker, B.G.; Amato, M.P.; Banwell, B.; Barkhof, F.; Bebo, B.; Becher, B.; Bethoux, F.; et al. Spinal cord involvement in multiple sclerosis and neuromyelitis optica spectrum disorders. Lancet Neurol. 2019, 18, 185–197. [Google Scholar] [CrossRef]
- Misu, T.; Höftberger, R.; Fujihara, K.; Wimmer, I.; Takai, Y.; Nishiyama, S.; Nakashima, I.; Konno, H.; Bradl, M.; Garzuly, F.; et al. Presence of six different lesion types suggests diverse mechanisms of tissue injury in neuromyelitis optica. Acta Neuropathol. 2013, 125, 815–827. [Google Scholar] [CrossRef] [Green Version]
- Asavapanumas, N.; Ratelade, J.; Verkman, A.S. Unique neuromyelitis optica pathology produced in naïve rats by intracerebral administration of NMO-IgG. Acta Neuropathol. 2014, 127, 539–551. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, M.C.; Bennett, J.L.; Verkman, A.S. Treatment of neuromyelitis optica: state-of-the-art and emerging therapies. Nat. Rev. Neurol. 2014, 10, 493–506. [Google Scholar] [CrossRef]
- Ikeshima-Kataoka, H. Neuroimmunological Implications of AQP4 in Astrocytes. Int. J. Mol. Sci. 2016, 17, 1306. [Google Scholar] [CrossRef]
- Hinson, S.R.; Clift, I.C.; Luo, N.; Kryzer, T.J.; Lennon, V.A. Autoantibody-induced internalization of CNS AQP4 water channel and EAAT2 glutamate transporter requires astrocytic Fc receptor. Proc. Natl. Acad. Sci. USA 2017, 114, 5491–5496. [Google Scholar] [CrossRef] [Green Version]
- Lieury, A.; Chanal, M.; Androdias, G.; Reynolds, R.; Cavagna, S.; Giraudon, P.; Confavreux, C.; Nataf, S. Tissue remodeling in periplaque regions of multiple sclerosis spinal cord lesions. Glia 2014, 62, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Nataf, S. Cord–Age–Gender Connections Shape the Pathophysiology of Multiple Sclerosis Progressive Forms. Int. J. Mol. Sci. 2019, 20, 5103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nataf, S.; Barritault, M.; Pays, L. A Unique TGFB1-Driven Genomic Program Links Astrocytosis, Low-Grade Inflammation and Partial Demyelination in Spinal Cord Periplaques from Progressive Multiple Sclerosis Patients. Int. J. Mol. Sci. 2017, 18, 2097. [Google Scholar] [CrossRef] [PubMed]
- Nataf, S.; Guillen, M.; Pays, L. TGFB1-Mediated Gliosis in Multiple Sclerosis Spinal Cords Is Favored by the Regionalized Expression of HOXA5 and the Age-Dependent Decline in Androgen Receptor Ligands. Int. J. Mol. Sci. 2019, 20, 5934. [Google Scholar] [CrossRef] [Green Version]
- Baghdassarian, D.; Toru-Delbauffe, D.; Gavaret, J.M.; Pierre, M. Effects of transforming growth factor-?1 on the extracellular matrix and cytoskeleton of cultured astrocytes. Glia 1993, 7, 193–202. [Google Scholar] [CrossRef]
- Baror, R.; Neumann, B.; Segel, M.; Chalut, K.J.; Fancy, S.P.J.; Schafer, D.P.; Franklin, R.J.M. Transforming growth factor-beta renders ageing microglia inhibitory to oligodendrocyte generation by CNS progenitors. Glia 2019, 67, 1374–1384. [Google Scholar] [CrossRef] [Green Version]
- Paul, D.; Dixit, A.; Srivastava, A.; Tripathi, M.; Prakash, D.; Sarkar, C.; Ramanujam, B.; Banerjee, J.; Chandra, P.S. Altered transforming growth factor beta/SMAD3 signalling in patients with hippocampal sclerosis. Epilepsy Res. 2018, 146, 144–150. [Google Scholar] [CrossRef]
- Das, A.; Wallace, G.C.; Holmes, C.; McDowell, M.L.; Smith, J.A.; Marshall, J.D.; Bonilha, L.; Edwards, J.C.; Glazier, S.S.; Ray, S.K.; et al. Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience 2012, 220, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Saadoun, S.; Bell, B.A.; Verkman, A.S.; Papadopoulos, M.C. Greatly improved neurological outcome after spinal cord compression injury in AQP4-deficient mice. Brain 2008, 131, 1087–1098. [Google Scholar] [CrossRef] [Green Version]
- Yong, Y.-X.; Li, Y.-M.; Lian, J.; Luo, C.-M.; Zhong, D.-X.; Han, K. Inhibitory role of lentivirus-mediated aquaporin-4 gene silencing in the formation of glial scar in a rat model of traumatic brain injury. J. Cell. Biochem. 2019, 120, 368–379. [Google Scholar] [CrossRef]
- Bramow, S.; Frischer, J.M.; Lassmann, H.; Koch-Henriksen, N.; Lucchinetti, C.F.; Sørensen, P.S.; Laursen, H. Demyelination versus remyelination in progressive multiple sclerosis. Brain 2010, 133, 2983–2998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frischer, J.M.; Weigand, S.D.; Guo, Y.; Kale, N.; Parisi, J.E.; Pirko, I.; Mandrekar, J.; Bramow, S.; Metz, I.; Brück, W.; et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann. Neurol. 2015, 78, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Oklinski, M.K.; Lim, J.-S.; Choi, H.-J.; Oklinska, P.; Skowronski, M.T.; Kwon, T.-H. Immunolocalization of Water Channel Proteins AQP1 and AQP4 in Rat Spinal Cord. J. Histochem. Cytochem. 2014, 62, 598–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oklinski, M.; Skowronski, M.; Skowronska, A.; Rützler, M.; Nørgaard, K.; Nieland, J.; Kwon, T.-H.; Nielsen, S. Aquaporins in the Spinal Cord. Int. J. Mol. Sci. 2016, 17, 2050. [Google Scholar] [CrossRef] [Green Version]
- Adler, P.; Kolde, R.; Kull, M.; Tkachenko, A.; Peterson, H.; Reimand, J.; Vilo, J. Mining for coexpression across hundreds of datasets using novel rank aggregation and visualization methods. Genome Biol. 2009, 10, R139. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Feng, Y.; Wu, J.; Song, Y.; Li, H.; Shen, Q.; Li, D.; Zhang, J.; Lu, Z.; Xiao, H.; et al. Metformin attenuates angiotensin II-induced TGFβ1 expression by targeting hepatocyte nuclear factor-4-α. Br. J. Pharmacol. 2018, 175, 1217–1229. [Google Scholar] [CrossRef]
- Chua, C.C.; Diglio, C.A.; Siu, B.B.; Chua, B.H.L. Angiotensin II induces TGF-β1 production in rat heart endothelial cells. Biochim. Biophys. Acta Mol. Cell Res. 1994, 1223, 141–147. [Google Scholar] [CrossRef]
- Wang, T.N.; Chen, X.; Li, R.; Gao, B.; Mohammed-Ali, Z.; Lu, C.; Yum, V.; Dickhout, J.G.; Krepinsky, J.C. SREBP-1 Mediates Angiotensin II-Induced TGF- β 1 Upregulation and Glomerular Fibrosis. J. Am. Soc. Nephrol. 2015, 26, 1839–1854. [Google Scholar] [CrossRef] [Green Version]
- Weigert, C.; Brodbeck, K.; Klopfer, K.; Häring, H.; Schleicher, E. Angiotensin II induces human TGF-β1 promoter activation: Similarity to hyperglycaemia. Diabetologia 2002, 45, 890–898. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Vita, J.; Sánchez-López, E.; Esteban, V.; Rupérez, M.; Egido, J.; Ruiz-Ortega, M. Angiotensin II Activates the Smad Pathway in Vascular Smooth Muscle Cells by a Transforming Growth Factor-β–Independent Mechanism. Circulation 2005, 111, 2509–2517. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Wang, B.; Jones, S.C.; Jassal, D.S.; Dixon, I.M.C. Interaction between angiotensin II and Smad proteins in fibroblasts in failing heart and in vitro. Am. J. Physiol. Circ. Physiol. 2000, 279, H3020–H3030. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, X.R.; Canlas, E.; Oka, K.; Truong, L.D.; Deng, C.; Bhowmick, N.A.; Ju, W.; Bottinger, E.P.; Lan, H.Y. Essential Role of Smad3 in Angiotensin II–Induced Vascular Fibrosis. Circ. Res. 2006, 98, 1032–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Füchtbauer, L.; Groth-Rasmussen, M.; Holm, T.H.; Løbner, M.; Toft-Hansen, H.; Khorooshi, R.; Owens, T. Angiotensin II Type 1 receptor (AT1) signaling in astrocytes regulates synaptic degeneration-induced leukocyte entry to the central nervous system. Brain Behav. Immun. 2011, 25, 897–904. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nataf, S. The Demonstration of an Aqp4/Tgf-Beta 1 Pathway in Murine Astrocytes Holds Implications for Both Neuromyelitis Optica and Progressive Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 1035. https://doi.org/10.3390/ijms21031035
Nataf S. The Demonstration of an Aqp4/Tgf-Beta 1 Pathway in Murine Astrocytes Holds Implications for Both Neuromyelitis Optica and Progressive Multiple Sclerosis. International Journal of Molecular Sciences. 2020; 21(3):1035. https://doi.org/10.3390/ijms21031035
Chicago/Turabian StyleNataf, Serge. 2020. "The Demonstration of an Aqp4/Tgf-Beta 1 Pathway in Murine Astrocytes Holds Implications for Both Neuromyelitis Optica and Progressive Multiple Sclerosis" International Journal of Molecular Sciences 21, no. 3: 1035. https://doi.org/10.3390/ijms21031035