Artificial Neural Network Approach for Modelling of Mercury Ions Removal from Water Using Functionalized CNTs with Deep Eutectic Solvent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hybrid Material Characterization
2.2. pH Effect
2.3. Initial Concentration Effect
2.4. Effect of Adsorbent Dosage
2.5. Kinetic Study
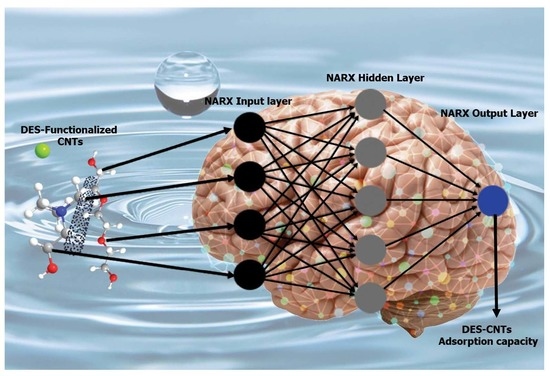
2.6. Neural Network Performance
3. Materials and Methods
3.1. Chemicals and Experiments Setup
3.2. Artificial Neural Networks (ANNs)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, H.-R.; Chen, C.-C.; Reddy, A.S.; Chen, C.-Y.; Li, W.R.; Tseng, M.-J.; Liu, H.-T.; Pan, W.; Maity, J.P.; Atla, S.B. Removal of mercury by foam fractionation using surfactin, a biosurfactant. Int. J. Mol. Sci. 2011, 12, 8245–8258. [Google Scholar] [CrossRef] [PubMed]
- Davodi, B.; Ghorbani, M.; Jahangiri, M. Adsorption of mercury from aqueous solution on synthetic polydopamine nanocomposite based on magnetic nanoparticles using Box–Behnken design. J. Taiwan Inst. Chem. Eng. 2017, 80, 363–378. [Google Scholar] [CrossRef]
- Khairi, N.; Yusof, N.; Abdullah, A.; Mohammad, F. Removal of toxic mercury from petroleum oil by newly synthesized molecularly-imprinted polymer. Int. J. Mol. Sci. 2015, 16, 10562–10577. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Ren, L.; Zhu, W.; Xu, Y.; Qian, X. Removal of mercury from aqueous solution using mesoporous silica nanoparticles modified with polyamide receptor. J. Colloid Interface Sci. 2015, 458, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.; Bridges, C. Chronic kidney disease and exposure to nephrotoxic metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar]
- Hsi, H.-C.; Lee, H.-H.; Hwang, J.-F.; Chen, W. Mercury speciation and distribution in a 660-megawatt utility boiler in Taiwan firing bituminous coals. J. Air Waste Manag. Assoc. 2010, 60, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Das, A.R.; Guha, A.K. A study on the adsorption mechanism of mercury on Aspergillus versicolor biomass. Environ. Sci. Technol. 2007, 41, 8281–8287. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.-B.; Shi, J.-B.; Feng, X.-B. Mercury pollution in China. Environ. Sci. Technol. 2006, 40, 3672–3678. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-X.; Feng-Ying, Z.; Yang, H.; Jian-Cong, N. Thorough removal of inorganic and organic mercury from aqueous solutions by adsorption on Lemna minor powder. J. Hazard. Mater. 2011, 186, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Huang, M.; Fu, T.; Pan, L.; Yao, W.; Guo, L. Microfiltration process by inorganic membranes for clarification of Tongbi liquor. Molecules 2012, 17, 1319–1334. [Google Scholar] [CrossRef]
- Chojnacki, A.; Chojnacka, K.; Hoffmann, J.; Gorecki, H. The application of natural zeolites for mercury removal: From laboratory tests to industrial scale. Miner. Eng. 2004, 17, 933–937. [Google Scholar] [CrossRef]
- Huttenloch, P.; Roehl, K.E.; Czurda, K. Use of copper shavings to remove mercury from contaminated groundwater or wastewater by amalgamation. Environ. Sci. Technol. 2003, 37, 4269–4273. [Google Scholar] [CrossRef] [PubMed]
- Biester, H.; Schuhmacher, P.; Müller, G. Effectiveness of mossy tin filters to remove mercury from aqueous solution by Hg (II) reduction and Hg (0) amalgamation. Water Res. 2000, 34, 2031–2036. [Google Scholar] [CrossRef]
- Oehmen, A.; Viegas, R.; Velizarov, S.; Reis, M.A.; Crespo, J.G. Removal of heavy metals from drinking water supplies through the ion exchange membrane bioreactor. Desalination 2006, 199, 405–407. [Google Scholar] [CrossRef]
- Kyzas, G.; Deliyanni, E. Mercury (II) removal with modified magnetic chitosan adsorbents. Molecules 2013, 18, 6193–6214. [Google Scholar] [CrossRef] [PubMed]
- Oubagaranadin, J.U.K.; Sathyamurthy, N.; Murthy, Z. Evaluation of Fuller’s earth for the adsorption of mercury from aqueous solutions: A comparative study with activated carbon. J. Hazard. Mater. 2007, 142, 165–174. [Google Scholar] [CrossRef]
- Lourie, E.; Gjengedal, E. Metal sorption by peat and algae treated peat: Kinetics and factors affecting the process. Chemosphere 2011, 85, 759–764. [Google Scholar] [CrossRef]
- Azari, A.; Gharibi, H.; Kakavandi, B.; Ghanizadeh, G.; Javid, A.; Mahvi, A.H.; Sharafi, K.; Khosravia, T. Magnetic adsorption separation process: An alternative method of mercury extracting from aqueous solution using modified chitosan coated Fe3O4 nanocomposites. J. Chem. Technol. Biotechnol. 2017, 92, 188–200. [Google Scholar] [CrossRef]
- Davis, A.P. Hazardous and industrial wastes. In Proceedings of the Twenty-Fifth Mid-Atlantic Industrial Waste Conference, College Park, MD, USA, 7–9 July 1993; Technomic Publishing Co. Inc.: Lancaster, PA, USA, 1993. [Google Scholar]
- Keppert, M.; Doušová, B.; Reiterman, P.; Koloušek, D.; Záleská, M.; Černý, R. Application of heavy metals sorbent as reactive component in cementitious composites. J. Clean. Prod. 2018, 199, 565–573. [Google Scholar] [CrossRef]
- Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar]
- Tawabini, B.S.; Al-Khaldi, S.F.; Khaled, M.M.; Atieh, M.A. Removal of arsenic from water by iron oxide nanoparticles impregnated on carbon nanotubes. J. Environ. Sci. Health Part A 2011, 46, 215–223. [Google Scholar] [CrossRef]
- Lu, M.; Ohba, T.; Kaneko, K.; Hata, K.; Yumura, M.; Iijima, S.; Komatsu, H.; Sakuma, A.; Kanoh, H. Electron density modification of single wall carbon nanotubes (SWCNT) by liquid-phase molecular adsorption of hexaiodobenzene. Materials 2013, 6, 535–543. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Fu, K.; Lin, Y.; Huang, W. Functionalized carbon nanotubes: Properties and applications. Acc. Chem. Res. 2002, 35, 1096–1104. [Google Scholar] [CrossRef]
- Fiyadh, S.S.; AlSaadi, M.A.; AlOmar, M.K.; Fayaed, S.S.; Mjalli, F.S.; El-Shafie, A. BTPC-Based DES-Functionalized CNTs for A s 3+ Removal from Water: NARX Neural Network Approach. J. Environ. Eng. 2018, 144, 04018070. [Google Scholar] [CrossRef]
- Garcia, G.; Atilhan, M.; Aparicio, S. Interfacial properties of deep eutectic solvents regarding to CO2 capture. J. Phys. Chem. C 2015, 119, 21413–21425. [Google Scholar] [CrossRef]
- Garcia, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep eutectic solvents: Physicochemical properties and gas separation applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible deep eutectic solvents based on choline chloride: Characterization and application to the extraction of rutin from Sophora japonica. Acs Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Zhou, C.; Ding, L.; Skibniewski, M.J.; Luo, H.; Zhang, H. Data based complex network modeling and analysis of shield tunneling performance in metro construction. Adv. Eng. Inform. 2018, 38, 168–186. [Google Scholar] [CrossRef]
- Fiyadh, S.S.; AlSaadi, M.A.; AlOmar, M.K.; Fayaed, S.S.; Hama, A.R.; Bee, S.; El-Shafie, A. The modelling of lead removal from water by deep eutectic solvents functionalized CNTs: Artificial neural network (ANN) approach. Water Sci. Technol. 2017, 76, 2413–2426. [Google Scholar] [CrossRef]
- Fiyadh, S.S.; AlSaadi, M.A.; AlOmar, M.K.; Fayaed, S.S.; El-Shafie, A. Lead removal from water using DES functionalized CNTs: ANN modeling approach. Desalin. Water Treat. 2019, 150, 105–113. [Google Scholar] [CrossRef]
- Tanzifi, M.; Yaraki, M.T.; Kiadehi, A.D.; Hosseini, S.H.; Olazar, M.; Bharti, A.K.; Agarwal, S.; Gupta, V.K.; Kazemi, A. Adsorption of Amido Black 10B from aqueous solution using polyaniline/SiO2 nanocomposite: Experimental investigation and artificial neural network modeling. J. Colloid Interface Sci. 2018, 510, 246–261. [Google Scholar] [CrossRef]
- Fiyadh, S.S.; AlSaadi, M.A.; Jaafar, W.Z.B.; AlOmar, M.K.; Fayaed, S.S.; Hama, A.R.; Hin, L.S.; El-Shafie, A. Mercury removal from water using deep eutectic solvents-functionalized multi walled carbon nanotubes: Nonlinear autoregressive network with an exogenous input neural network approach. Environ. Prog. Sustain. 2019. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Alsaadi, M.A.; Hayyan, M.; Akib, S.; Ibrahim, M.; Hashim, M.A. Allyl triphenyl phosphonium bromide based DES-functionalized carbon nanotubes for the removal of mercury from water. Chemosphere 2017, 167, 44–52. [Google Scholar] [CrossRef]
- Kazemipour, M.; Ansari, M.; Tajrobehkar, S.; Majdzadeh, M.; Kermani, H.R. Removal of lead, cadmium, zinc, and copper from industrial wastewater by carbon developed from walnut, hazelnut, almond, pistachio shell, and apricot stone. J. Hazard. Mater. 2008, 150, 322–327. [Google Scholar] [CrossRef]
- Witek-Krowiak, A.; Szafran, R.G.; Modelski, S. Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent. Desalination 2011, 265, 126–134. [Google Scholar] [CrossRef]
- Zabihi, M.; Asl, A.H.; Ahmadpour, A. Studies on adsorption of mercury from aqueous solution on activated carbons prepared from walnut shell. J. Hazard. Mater. 2010, 174, 251–256. [Google Scholar] [CrossRef]
- Bandaru, N.M.; Reta, N.; Dalal, H.; Ellis, A.V.; Shapter, J.; Voelcker, N.H. Enhanced adsorption of mercury ions on thiol derivatized single wall carbon nanotubes. J. Hazard. Mater. 2013, 261, 534–541. [Google Scholar] [CrossRef]
- Das, B.; Mondal, N.; Bhaumik, R.; Roy, P. Insight into adsorption equilibrium, kinetics and thermodynamics of lead onto alluvial soil. Int. J. Environ. Sci. Technol. 2014, 11, 1101–1114. [Google Scholar] [CrossRef]
- Zhang, C.; Sui, J.; Li, J.; Tang, Y.; Cai, W. Efficient removal of heavy metal ions by thiol-functionalized superparamagnetic carbon nanotubes. Chem. Eng. J. 2012, 210, 45–52. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Hayyan, M.; Alsaadi, M.A.; Akib, S.; Hayyan, A.; Hashim, M.A. Glycerol-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2016, 215, 98–103. [Google Scholar] [CrossRef]
- AlSaadi, M.A.; Al Mamun, A.; Alam, M.Z.; Amosa, M.K.; Atieh, M.A. Removal of cadmium from water by CNT–PAC composite: Effect of functionalization. Nano 2016, 11, 1650011. [Google Scholar] [CrossRef]
- Wunsch, A.; Liesch, T.; Broda, S. Forecasting groundwater levels using nonlinear autoregressive networks with exogenous input (NARX). J. Hydrol. 2018, 567, 743–758. [Google Scholar] [CrossRef]
- Fayaed, S.S.; El-Shafie, A.; Jaafar, O. Adaptive neuro-fuzzy inference system–based model for elevation–surface area–storage interrelationships. Neural Comput. Appl. 2013, 22, 987–998. [Google Scholar] [CrossRef]
- Samarasinghe, S. Neural Networks for Applied Sciences and Engineering: From Fundamentals to Complex Pattern Recognition; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Govindaraju, R.S. Artificial neural networks in hydrology. I: Preliminary concepts. J. Hydrol. Eng. 2000, 5, 115–123. [Google Scholar]
- Chen, S.; Billings, S.; Grant, P. Non-linear system identification using neural networks. Int. J. Control 1990, 51, 1191–1214. [Google Scholar] [CrossRef]
- El-Shafie, A.H.; El-Manadely, M.S. An integrated neural network stochastic dynamic programming model for optimizing the operation policy of Aswan High Dam. Hydrol. Res. 2011, 42, 50–67. [Google Scholar] [CrossRef]
- El-Shafie, A.; Taha, M.R.; Noureldin, A. A neuro-fuzzy model for inflow forecasting of the Nile river at Aswan high dam. Water Resour. Manag. 2007, 21, 533–556. [Google Scholar] [CrossRef]
- Olaofe, Z.O. A 5-day wind speed & power forecasts using a layer recurrent neural network (LRNN). Sustain. Energy Technol. Assess. 2014, 6, 1–24. [Google Scholar]









| Pseudo First-Order ln(qe–qt) vs. Time (t) | Pseudo Second-Order (t/qt vs. t) | Intraparticle (qt vs. t0.5) | ||||||
|---|---|---|---|---|---|---|---|---|
| Dose mg | pH | C0 mg/L | Experimental R2 | NARX output R2 | Experimental R2 | NARX output R2 | Experimental R2 | NARX output R2 |
| 20 | 3 | 3 | 0.9378 | 0.9314 | 0.9978 | 0.9971 | 0.9114 | 0.9128 |
| 20 | 6 | 5 | 0.8689 | 0.8301 | 0.9959 | 0.9918 | 0.9053 | 0.9058 |
| 30 | 6 | 5 | 0.5692 | 0.5489 | 0.9969 | 0.9946 | 0.6045 | 0.6721 |
| Indicator | NARX | FFNN | LR |
|---|---|---|---|
| MSE | 1.15 × 10−3 | 2.27 × 10−3 | 2.2 × 10−3 |
| RMSE | 3.40 × 10−2 | 4.81 × 10−2 | 4.74 × 10−2 |
| RRMSE | 6.24 × 10−2 | 7.92 × 10−2 | 7.61 × 10−2 |
| MAPE | 5.71% | 6.92% | 6.68% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiyadh, S.S.; AlOmar, M.K.; Binti Jaafar, W.Z.; AlSaadi, M.A.; Fayaed, S.S.; Binti Koting, S.; Lai, S.H.; Chow, M.F.; Ahmed, A.N.; El-Shafie, A. Artificial Neural Network Approach for Modelling of Mercury Ions Removal from Water Using Functionalized CNTs with Deep Eutectic Solvent. Int. J. Mol. Sci. 2019, 20, 4206. https://doi.org/10.3390/ijms20174206
Fiyadh SS, AlOmar MK, Binti Jaafar WZ, AlSaadi MA, Fayaed SS, Binti Koting S, Lai SH, Chow MF, Ahmed AN, El-Shafie A. Artificial Neural Network Approach for Modelling of Mercury Ions Removal from Water Using Functionalized CNTs with Deep Eutectic Solvent. International Journal of Molecular Sciences. 2019; 20(17):4206. https://doi.org/10.3390/ijms20174206
Chicago/Turabian StyleFiyadh, Seef Saadi, Mohamed Khalid AlOmar, Wan Zurina Binti Jaafar, Mohammed Abdulhakim AlSaadi, Sabah Saadi Fayaed, Suhana Binti Koting, Sai Hin Lai, Ming Fai Chow, Ali Najah Ahmed, and Ahmed El-Shafie. 2019. "Artificial Neural Network Approach for Modelling of Mercury Ions Removal from Water Using Functionalized CNTs with Deep Eutectic Solvent" International Journal of Molecular Sciences 20, no. 17: 4206. https://doi.org/10.3390/ijms20174206