Amelioration of Enterotoxigenic Escherichia coli-Induced Intestinal Barrier Disruption by Low-Molecular-Weight Chitosan in Weaned Pigs is Related to Suppressed Intestinal Inflammation and Apoptosis
Abstract
:1. Introduction
2. Results
2.1. Growth Performance
2.2. Serum Indices
2.3. Intestinal Integrity
2.4. Intestinal Cytokine Content
2.5. Intestinal Mast Cell Protease Content
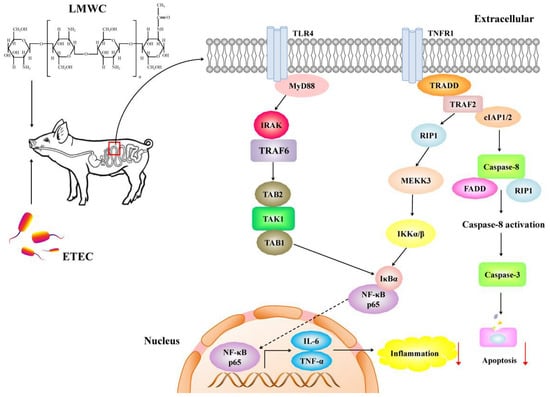
2.6. Intestinal Toll-like Receptor 4 (TLR4) Signalling Pathway-Related Gene Expression
2.7. Intestinal Nuclear Factor-κB (NF-κB) p65 Protein Abundance
2.8. Enterocyte Apoptosis Percentage
2.9. Enterocyte Apoptosis-Related Gene Expression
2.10. Intestinal Cleaved Cysteinyl Aspartate-Specific Protease (Caspase) Contents
3. Discussion
4. Materials and Methods
4.1. Animals and Feeding Management
4.2. Experimental Design
4.3. Sample Collection
4.4. Serum Parameter Measurements
4.5. Immunofluorescence Assay
4.6. Western Blot Analysis
4.7. Intestinal Cytokine and Cleaved Caspase Content Determinations
4.8. Immunohistochemical Analysis
4.9. Enterocyte Apoptosis Assessment
4.10. RNA Isolation, cDNA Synthesis and qPCR
4.11. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wijtten, P.J.A.; van der Meulen, J.; Verstegen, M.W.A. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.E.; Grabinger, T.; Brunner, T. Cell death at the intestinal epithelial front line. FEBS J. 2016, 283, 2701–2719. [Google Scholar] [CrossRef] [PubMed]
- Dubreuil, J.D. Enterotoxigenic Escherichia coli targeting intestinal epithelial tight junctions: An effective way to alter the barrier integrity. Microb. Pathog. 2017, 113, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; Kaushik, R.S.; Hardwidge, P.R. Disruption of transepithelial resistance by enterotoxigenic Escherichia coli. Vet. Microbiol. 2010, 141, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Mukiza, C.N.; Dubreuil, J.D. Escherichia coli heat-stable toxin b impairs intestinal epithelial barrier function by altering tight junction proteins. Infect. Immun. 2013, 81, 2819–2827. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Britti, M.S.; Konstantinov, S.R.; Smidt, H.; de Vos, W.M.; Mengheri, E. The novel porcine Lactobacillus sobrius strain protects intestinal cells from enterotoxigenic Escherichia coli K88 infection and prevents membrane barrier damage. J. Nutr. 2007, 137, 2709–2716. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Garaguso, I.; Britti, M.S.; Mengheri, E. Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli. J. Nutr. 2003, 133, 4077–4082. [Google Scholar] [CrossRef]
- Xia, Y.Y.; Bin, P.; Liu, S.J.; Chen, S.; Yin, J.; Liu, G.; Tang, Z.Y.; Ren, W.K. Enterotoxigenic Escherichia coli infection promotes apoptosis in piglets. Microb. Pathog. 2018, 125, 290–294. [Google Scholar] [CrossRef]
- Negroni, A.; Cucchiara, S.; Stronati, L. Apoptosis, necrosis, and necroptosis in the gut and intestinal homeostasis. Mediat. Inflamm. 2015, 2015. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.G.; Liu, N.; Liu, C.S.; Liu, C.G.; Meng, X.H.; Yu, L.J.; Kenendy, J.F. Physicochemical characterization and antibacterial property of chitosan acetates. Carbohydr. Polym. 2007, 67, 227–232. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Davoodbasha, M.A.; Lee, S.Y.; Kim, J.W. Solution plasma mediated formation of low molecular weight chitosan and its application as a biomaterial. Int. J. Biol. Macromol. 2018, 118, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Du, Y.G.; Zhang, J.Z. Low molecular weight and oligomeric chitosans and their bioactivities. Curr. Top. Med. Chem. 2009, 9, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.L.; Wang, Y.; Wen, X.L.; Wang, L.; Jiang, Z.Y.; Zheng, C.T. Effects of low-molecular-weight chitosan on the growth performance, intestinal morphology, barrier function, cytokine expression and antioxidant system of weaned piglets. BMC Vet. Res. 2018, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Van Itallie, C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. -Gastrointest. Liver Physiol. 1995, 269, G467–G475. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef]
- Förster, C. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 2008, 130, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.M.; Jiang, Z.Y.; Zheng, C.T.; Wang, L.; Yang, X.F. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 2014, 92, 1496–1503. [Google Scholar] [CrossRef]
- Che, L.Q.; Xu, Q.; Wu, C.; Luo, Y.H.; Huang, X.B.; Zhang, B.; Auclair, E.; Kiros, T.; Fang, Z.F.; Lin, Y. Effects of dietary live yeast supplementation on growth performance, diarrhoea severity, intestinal permeability and immunological parameters of weaned piglets challenged with enterotoxigenic Escherichia coli K88. Br. J. Nutr. 2017, 118, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S. Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.M.; Skare, I.B.; Stankewich, M.C.; Furuse, M.; Tsukita, S.; Rogers, R.A.; Lynch, R.D.; Schneeberger, E.E. Occludin is a functional component of the tight junction. J. Cell Sci. 1996, 109, 2287–2298. [Google Scholar] [PubMed]
- Fukudome, I.; Kobayashi, M.; Dabanaka, K.; Maeda, H.; Okamoto, K.; Okabayashi, T.; Baba, R.; Kumagai, N.; Oba, K.; Fujita, M. Diamine oxidase as a marker of intestinal mucosal injury and the effect of soluble dietary fiber on gastrointestinal tract toxicity after intravenous 5-fluorouracil treatment in rats. Med Mol. Morphol. 2014, 47, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.; Lindholt, J.S.; Erlandsen, E.J.; Mortensen, F.V. d-Lactate as a marker of venous-induced intestinal ischemia: An experimental study in pigs. Int. J. Surg. 2011, 9, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. 2009, 14, 2765–2778. [Google Scholar] [CrossRef]
- Xu, C.L.; Wang, Y.M.; Sun, R.; Qiao, X.J.; Shang, X.Y.; Niu, W.N. Modulatory effects of vasoactive intestinal peptide on intestinal mucosal immunity and microbial community of weaned piglets challenged by an enterotoxigenic Escherichia coli (K88). PLoS ONE 2014, 9, e104183. [Google Scholar] [CrossRef]
- Wu, Y.P.; Zhu, C.; Chen, Z.; Chen, Z.J.; Zhang, W.N.; Ma, X.Y.; Wang, L.; Yang, X.F.; Jiang, Z.Y. Protective effects of Lactobacillus plantarum on epithelial barrier disruption caused by enterotoxigenic Escherichia coli in intestinal porcine epithelial cells. Vet. Immunol. Immunopathol. 2016, 172, 55–63. [Google Scholar] [CrossRef]
- Abraham, S.N.; John, A.L.S. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.; Guilarte, M.; Alonso, C.; Malagelada, J.R. Pathogenesis of irritable bowel syndrome: The mast cell connection. Scand. J. Gastroenterol. 2005, 40, 129–140. [Google Scholar] [CrossRef]
- McDermott, J.R.; Bartram, R.E.; Knight, P.A.; Miller, H.R.; Garrod, D.R.; Grencis, R.K. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc. Natl. Acad. Sci. USA 2003, 100, 7761–7766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caughey, G.H. Mast cell tryptases and chymases in inflammation and host defense. Immunol. Rev. 2007, 217, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Yang, P.C.; Darmoul, D.; Amadesi, S.; Saito, T.; Cottrell, G.S.; Coelho, A.M.; Singh, P.; Grady, E.F.; Perdue, M. Mast Cell Tryptase Controls Paracellular Permeability of the Intestine role of protease-activated receptor 2 and β-arrestins. J. Biol. Chem. 2005, 280, 31936–31948. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Miner Jr, P.B. The role of mast cells in common gastrointestinal diseases. Curr. Allergy Asthma Rep. 2004, 4, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.K.; Zhu, H.L.; Wu, Z.F.; Hou, Y.Q. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Xu, X.; Wang, X.Y.; Wu, H.T.; Zhu, H.L.; Hou, Y.Q.; Dai, B.; Liu, X.T.; Liu, Y.L. Glutamate alleviates intestinal injury, maintains mTOR and suppresses TLR4 and NOD signaling pathways in weanling pigs challenged with lipopolysaccharide. Sci. Rep. 2018, 8, 15124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.L.; Ghosh, S. Negative regulation of toll-like receptor-mediated signaling by Tollip. J. Biol. Chem. 2002, 277, 7059–7065. [Google Scholar] [CrossRef]
- Wu, W.S.; Wang, Y.L.; Zou, J.J.; Long, F.; Yan, H.H.; Zeng, L.J.; Chen, Y.B. Bifidobacterium adolescentis protects against necrotizing enterocolitis and upregulates TOLLIP and SIGIRR in premature neonatal rats. BMC Pediatrics 2017, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.A.; Gores, G.J. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am. J. Physiol. -Gastrointest. Liver Physiol. 1997, 273, G1174. [Google Scholar] [CrossRef]
- Günther, C.; Neumann, H.; Neurath, M.F.; Becker, C. Apoptosis, necrosis and necroptosis: Cell death regulation in the intestinal epithelium. Gut 2013, 62, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, Y.H.; Yang, J.C.; Yang, G.Y.; Zhou, D.; Wang, J.F. A selected Lactobacillus rhamnosus strain promotes EGFR-independent Akt activation in an enterotoxigenic Escherichia coli K88-infected IPEC-J2 cell model. PLoS ONE 2015, 10, e0125717. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Duprez, L.; Wirawan, E.; Berghe, T.V.; Vandenabeele, P. Major cell death pathways at a glance. Microbes Infect. 2009, 11, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat. Rev. Cancer 2002, 2, 420–430. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin. 2005, 55, 178–194. [Google Scholar] [CrossRef]
- National Reasearch Council. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Wan, J.; Zhang, J.; Chen, D.W.; Yu, B.; Mao, X.B.; Zheng, P.; Yu, J.; Huang, Z.Q.; Luo, J.Q.; Luo, Y.H. Alginate oligosaccharide alleviates enterotoxigenic Escherichia coli-induced intestinal mucosal disruption in weaned pigs. Food Funct. 2018, 9, 6401–6413. [Google Scholar] [CrossRef]
- Chen, H.; Mao, X.B.; He, J.; Yu, B.; Huang, Z.Q.; Yu, J.; Zheng, P.; Chen, D.W. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br. J. Nutr. 2013, 110, 1837–1848. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]










| Ingredient | Content (%) |
|---|---|
| Corn (7.8% crude protein) | 29.00 |
| Extruded corn (7.8% crude protein) | 24.00 |
| Soybean meal (44.2% crude protein) | 11.00 |
| Extruded soybean | 10.00 |
| Whey powder (low protein) | 7.00 |
| Fish meal (62.5% crude protein) | 6.00 |
| Soybean protein concentrate | 4.00 |
| Sucrose | 3.00 |
| Glucose | 2.00 |
| Soybean oil | 1.50 |
| Limestone | 0.70 |
| Dicalcium phosphate | 0.48 |
| l-Lysine·HCl (78%) | 0.35 |
| NaCl | 0.30 |
| Chloride choline | 0.15 |
| dl-Methionine | 0.15 |
| l-Threonine (98.5%) | 0.10 |
| Tryptophan (98%) | 0.02 |
| Vitamin premix * | 0.05 |
| Mineral premix † | 0.20 |
| Total | 100 |
| Calculated composition | |
| Digestible energy (MJ kg−1) | 14.85 |
| Crude protein | 19.60 |
| Calcium | 0.85 |
| Total phosphorus | 0.57 |
| Available phosphorus | 0.46 |
| Lysine | 1.39 |
| Methionine | 0.48 |
| Methionine + Cysteine | 0.75 |
| Threonine | 0.84 |
| Tryptophan | 0.22 |
| Gene * | Primer Sequence (5′–3′) | Size (bp) | Accession No. |
|---|---|---|---|
| TLR4 | Forward: TCAGTTCTCACCTTCCTCCTG | 166 | NM_001113039.2 |
| Reverse: GTTCATTCCTCACCCAGTCTTC | |||
| MyD88 | Forward: GATGGTAGCGGTTGTCTCTGAT | 148 | NM_001099923.1 |
| Reverse: GATGCTGGGGAACTCTTTCTTC | |||
| IRAK1 | Forward: CAAGGCAGGTCAGGTTTCGT | 115 | XM_003135490.4 |
| Reverse: TTCGTGGGGCGTGTAGTGT | |||
| TRAF6 | Forward: CAAGAGAATACCCAGTCGCACA | 122 | NM_001105286.1 |
| Reverse: ATCCGAGACAAAGGGGAAGAA | |||
| Tollip | Forward: GCAGCAGCAACAGCAGAT | 133 | NM_001315800.1 |
| Reverse: GGTCACGCCGTAGTTCTTC | |||
| SIGIRR | Forward: ACCTTCACCTGCTCCATCCA | 205 | NM_001315689.1 |
| Reverse: TTCCGTCATTCATCTCCACCTC | |||
| Bcl-2 | Forward: AGCATGCGGCCTCTATTTGA | 120 | XM_021099593.1 |
| Reverse: GGCCCGTGGACTTCACTTAT | |||
| Bax | Forward: CTGACGGCAACTTCAACTGG | 200 | XM_003127290.5 |
| Reverse: CGTCCCAAAGTAGGAGAGGA | |||
| Fas | Forward: TGATGCCCAAGTGACTGACC | 103 | NM_213839.1 |
| Reverse: GCAGAATTGACCCTCACGAT | |||
| TNFR1 | Forward: CTGGCATTCTTCCTCTTCGTTG | 109 | NM_213969.1 |
| Reverse: CCGGCTCTCCCTCCTTTACA | |||
| TRADD | Forward: AGGCGTGCTTGGAGGCT | 124 | XM_021094047.1 |
| Reverse: GCGAAGATGAAATTCAAACAGC | |||
| FADD | Forward: CTGCGACAACGTGGGGA | 101 | NM_001031797.1 |
| Reverse: TCAGGTTTCGGGGATACTTC | |||
| GAPDH | Forward: ATGGTGAAGGTCGGAGTGAAC | 235 | NM_001206359.1 |
| Reverse: CTCGCTCCTGGAAGATGGT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, J.; Zhang, J.; Wu, G.; Chen, D.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Luo, J.; Mao, X.; et al. Amelioration of Enterotoxigenic Escherichia coli-Induced Intestinal Barrier Disruption by Low-Molecular-Weight Chitosan in Weaned Pigs is Related to Suppressed Intestinal Inflammation and Apoptosis. Int. J. Mol. Sci. 2019, 20, 3485. https://doi.org/10.3390/ijms20143485
Wan J, Zhang J, Wu G, Chen D, Yu B, Huang Z, Luo Y, Zheng P, Luo J, Mao X, et al. Amelioration of Enterotoxigenic Escherichia coli-Induced Intestinal Barrier Disruption by Low-Molecular-Weight Chitosan in Weaned Pigs is Related to Suppressed Intestinal Inflammation and Apoptosis. International Journal of Molecular Sciences. 2019; 20(14):3485. https://doi.org/10.3390/ijms20143485
Chicago/Turabian StyleWan, Jin, Jiao Zhang, Guozhong Wu, Daiwen Chen, Bing Yu, Zhiqing Huang, Yuheng Luo, Ping Zheng, Junqiu Luo, Xiangbing Mao, and et al. 2019. "Amelioration of Enterotoxigenic Escherichia coli-Induced Intestinal Barrier Disruption by Low-Molecular-Weight Chitosan in Weaned Pigs is Related to Suppressed Intestinal Inflammation and Apoptosis" International Journal of Molecular Sciences 20, no. 14: 3485. https://doi.org/10.3390/ijms20143485