RTA 408 Inhibits Interleukin-1β-Induced MMP-9 Expression via Suppressing Protein Kinase-Dependent NF-κB and AP-1 Activation in Rat Brain Astrocytes
Abstract
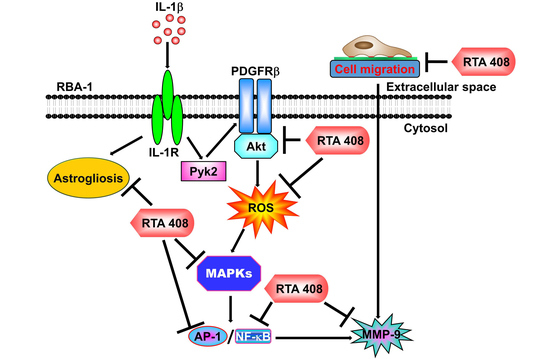
:1. Introduction
2. Results
2.1. RTA 408 Attenuates IL-1β-Induced MMP-9 Expression
2.2. RTA 408 Inhibits IL-1β-Stimulated Phosphorylation of Pyk2/PDGFR/Akt in RBA-1 Cells
2.3. RTA 408 Inhibits IL-1β-Stimulated ROS Generation in RBA-1 Cells
2.4. RTA 408 Inhibits IL-1β -Stimulated Phosphorylation of MAPKs in RBA-1 Cells
2.5. RTA 408 Inhibits IL-1β-Stimulated Activation of NF-κB and AP-1 in RBA-1 cells
2.6. RTA 408 Inhibits IL-1β-Induced Astroglial Cell Migration and Astrogliosis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Treatment
4.3. Protein Preparation and Western Blotting
4.4. MMP Gelatin Zymography
4.5. Total RNA Extraction and Real-Time PCR Analysis
4.6. Rat MMP-9 Promoter Construction, Transfection, and Luciferase Reporter Gene Assays
4.7. Isolation of Cell Fractions
4.8. Measurement of Intracellular ROS Accumulation
4.9. Immunofluorescence Staining
4.10. Cell Migration Assay
4.11. Chromatin Immunoprecipitation (ChIP) Assay
4.12. Statistical Analysis of Data
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AP-1 | activator protein 1 |
| BBB | blood-brain barrier |
| BCA | bicinchoninic acid |
| BK | bradykinin |
| BSA | bovine serum albumin |
| CDDO-Me | C-28 methyl ester of 2-cyano-3,12- dioxooleana-1,9 (11)-dien-28-oic acid |
| ChIP | chromatin immunoprecipitation |
| CNS | central nervous system |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | dimethyl sulfoxide |
| ECL | enhanced chemiluminescence |
| EDTA | ethylenediaminetetraacetic acid |
| ERK1/2 | extracellular signal-regulated kinase 1/2 |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GFAP | glial fibrillary acidic protein |
| GFP | green fluorescent protein |
| IKK | IκB-kinase |
| IL-1β | interleukin-1β |
| JNK | c-Jun amino-terminal kinase |
| MAPK | mitogen-activated protein kinase |
| MMP | matrix metalloproteinase |
| NF-κB | nuclear factor-κB |
| NOX | NADPH oxidase |
| PD | Parkinson’s disease |
| PDGFR | platelet-derived growth factor receptor |
| PI3K | phosphoinositide 3-kinase |
| PMSF | phenylmethylsulfonyl fluoride |
| Pyk2 | proline-rich tyrosine kinase |
| RBA-1 | rat brain astrocytes |
| ROS | reactive oxygen species |
| RTA 408 | N-(2-cyano-3,12-dioxo-28-noroleana-1,9(11)-dien-17-yl)-2-2-difluoropropanamide |
| SEM | standard error of the mean |
| SDS | Sodium dodecyl sulfate |
| TNF | tumor necrosis factor |
| XTT | sodium 3′-[1-[(phenylamino)-carbony]-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene-sulfonic acid hydrate |
References
- McGeer, P.L.; McGeer, E.G. Inflammation and the degenerative diseases of aging. Ann. N. Y. Acad. Sci. 2004, 1035, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Cebrian, C.; Loike, J.D.; Sulzer, D. Neuroinflammation in Parkinson’s disease animal models: A cell stress response or a step in neurodegeneration? Curr. Top Behav. Neurosci. 2015, 22, 237–270. [Google Scholar] [CrossRef] [PubMed]
- Dursun, E.; Gezen-Ak, D.; Hanagasi, H.; Bilgic, B.; Lohmann, E.; Ertan, S.; Atasoy, I.L.; Alaylioglu, M.; Araz, O.S.; Onal, B.; et al. The interleukin 1 alpha, interleukin 1 beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer’s disease, mild cognitive impairment or Parkinson’s disease. J. Neuroimmunol. 2015, 283, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.M.; Tyrrell, P.J.; Rothwell, N.J. Interleukin-1 and neuronal injury. Nat. Rev. Immunol. 2005, 5, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [PubMed]
- Choi, S.S.; Lee, H.J.; Lim, I.; Satoh, J.; Kim, S.U. Human astrocytes: Secretome profiles of cytokines and chemokines. PLoS ONE 2014, 9, e92325. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ochiai, K.; Abe, A.; Kishi, S.; Takayama, K.; Sunden, Y. Astrocytic growth through the autocrine/paracrine production of IL-1beta in the early infectious phase of fowl glioma-inducing virus. Avian Pathol. 2014, 43, 437–442. [Google Scholar] [CrossRef]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) pathway. Sci. Signal. 2010, 3. [Google Scholar] [CrossRef]
- Yang, C.M.; Hsieh, H.L.; Yu, P.H.; Lin, C.C.; Liu, S.W. IL-1beta Induces MMP-9-Dependent Brain Astrocytic Migration via Transactivation of PDGF Receptor/NADPH Oxidase 2-Derived Reactive Oxygen Species Signals. Mol. Neurobiol. 2015, 52, 303–317. [Google Scholar] [CrossRef]
- Kamat, P.K.; Swarnkar, S.; Rai, S.; Kumar, V.; Tyagi, N. Astrocyte mediated MMP-9 activation in the synapse dysfunction: An implication in Alzheimer disease. Ther. Targets Neurol. Dis. 2014, 1. [Google Scholar] [CrossRef]
- Park, K.P.; Rosell, A.; Foerch, C.; Xing, C.; Kim, W.J.; Lee, S.; Opdenakker, G.; Furie, K.L.; Lo, E.H. Plasma and brain matrix metalloproteinase-9 after acute focal cerebral ischemia in rats. Stroke 2009, 40, 2836–2842. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A. Matrix metalloproteinases in neuroinflammation. Glia 2002, 39, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Miller, F.; Cazaubon, S.; Couraud, P.O. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim. Biophys. Acta 2009, 1788, 842–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecil, G.G.; Larsen, P.H.; Corley, S.M.; Herx, L.M.; Besson, A.; Goodyer, C.G.; Yong, V.W. Interleukin-1 is a key regulator of matrix metalloproteinase-9 expression in human neurons in culture and following mouse brain trauma in vivo. J. Neurosci. Res. 2000, 61, 212–224. [Google Scholar] [CrossRef]
- Annese, V.; Herrero, M.T.; Di Pentima, M.; Gomez, A.; Lombardi, L.; Ros, C.M.; De Pablos, V.; Fernandez-Villalba, E.; De Stefano, M.E. Metalloproteinase-9 contributes to inflammatory glia activation and nigro-striatal pathway degeneration in both mouse and monkey models of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinsonism. Brain Struct. Funct. 2015, 220, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Moon, Y.J.; Seo, J.E.; Lee, Y.; Kim, K.H.; Chung, J.H. Reactive oxygen species produced by NADPH oxidase, xanthine oxidase, and mitochondrial electron transport system mediate heat shock-induced MMP-1 and MMP-9 expression. Free Radic. Biol. Med. 2008, 44, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Burda, J.E.; Sofroniew, M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef]
- Pekny, M.; Pekna, M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim. Biophys. Acta 2016, 1862, 483–491. [Google Scholar] [CrossRef]
- Niranjan, R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: Focus on astrocytes. Mol. Neurobiol. 2014, 49, 28–38. [Google Scholar] [CrossRef]
- NA, J.C.F.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; Andre, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22. [Google Scholar] [CrossRef]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Probst, B.L.; Trevino, I.; McCauley, L.; Bumeister, R.; Dulubova, I.; Wigley, W.C.; Ferguson, D.A. RTA 408, A Novel Synthetic Triterpenoid with Broad Anticancer and Anti-Inflammatory Activity. PLoS ONE 2015, 10, e0122942. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ward, K.; Xavier, C.; Jann, J.; Clark, A.F.; Pang, I.H.; Wu, H. The novel triterpenoid RTA 408 protects human retinal pigment epithelial cells against H2O2-induced cell injury via NF-E2-related factor 2 (Nrf2) activation. Redox Biol. 2016, 8, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Reisman, S.A.; Goldsberry, A.R.; Lee, C.Y.; O’Grady, M.L.; Proksch, J.W.; Ward, K.W.; Meyer, C.J. Topical application of RTA 408 lotion activates Nrf2 in human skin and is well-tolerated by healthy human volunteers. BMC Dermatol. 2015, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Reisman, S.A.; Lee, C.Y.; Meyer, C.J.; Proksch, J.W.; Ward, K.W. Topical application of the synthetic triterpenoid RTA 408 activates Nrf2 and induces cytoprotective genes in rat skin. Arch Dermatol. Res. 2014, 306, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Reisman, S.A.; Lee, C.Y.; Meyer, C.J.; Proksch, J.W.; Sonis, S.T.; Ward, K.W. Topical application of the synthetic triterpenoid RTA 408 protects mice from radiation-induced dermatitis. Radiat. Res. 2014, 181, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, P.S.; Ellison, T.; Waqas, B.; Sultan, D.; Abdou, S.; David, J.A.; Cohen, J.M.; Gomez-Viso, A.; Lam, G.; Kim, C.; et al. Targeted Nrf2 activation therapy with RTA 408 enhances regenerative capacity of diabetic wounds. Diabetes Res. Clin. Pract. 2018, 139, 11–23. [Google Scholar] [CrossRef]
- Han, P.; Qin, Z.; Tang, J.; Xu, Z.; Li, R.; Jiang, X.; Yang, C.; Xing, Q.; Qi, X.; Tang, M.; et al. RTA-408 Protects Kidney from Ischemia-Reperfusion Injury in Mice via Activating Nrf2 and Downstream GSH Biosynthesis Gene. Oxid. Med. Cell Longev. 2017, 2017, 7612182. [Google Scholar] [CrossRef]
- Shekh-Ahmad, T.; Eckel, R.; Dayalan Naidu, S.; Higgins, M.; Yamamoto, M.; Dinkova-Kostova, A.T.; Kovac, S.; Abramov, A.Y.; Walker, M.C. KEAP1 inhibition is neuroprotective and suppresses the development of epilepsy. Brain 2018, 141, 1390–1403. [Google Scholar] [CrossRef]
- Avraham, H.; Park, S.Y.; Schinkmann, K.; Avraham, S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000, 12, 123–133. [Google Scholar] [CrossRef]
- Yang, C.M.; Lee, I.T.; Hsu, R.C.; Chi, P.L.; Hsiao, L.D. NADPH oxidase/ROS-dependent PYK2 activation is involved in TNF-alpha-induced matrix metalloproteinase-9 expression in rat heart-derived H9c2 cells. Toxicol. Appl. Pharmacol. 2013, 272, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Berk, B.C. Transactivation: A novel signaling pathway from angiotensin II to tyrosine kinase receptors. J. Mol. Cell Cardiol. 2001, 33, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Demoulin, J.B.; Essaghir, A. PDGF receptor signaling networks in normal and cancer cells. Cytokine Growth Factor Rev. 2014, 25, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Toker, A.; Marmiroli, S. Signaling specificity in the Akt pathway in biology and disease. Adv. Biol. Regul. 2014, 55, 28–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.Y.; Hsieh, H.L.; Sun, C.C.; Tseng, C.P.; Yang, C.M. IL-1 beta induces proMMP-9 expression via c-Src-dependent PDGFR/PI3K/Akt/p300 cascade in rat brain astrocytes. J. Neurochem. 2008, 105, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Zhao, M.X.; Ren, X.S.; Liu, T.Y.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Wang, J.J.; Zhu, G.Q. Salusin-beta Promotes Vascular Smooth Muscle Cell Migration and Intimal Hyperplasia After Vascular Injury via ROS/NFkappaB/MMP-9 Pathway. Antioxid. Redox Signal. 2016, 24, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Compromised MAPK signaling in human diseases: An update. Arch Toxicol. 2015. [Google Scholar] [CrossRef]
- Wu, C.Y.; Hsieh, H.L.; Jou, M.J.; Yang, C.M. Involvement of p42/p44 MAPK, p38 MAPK, JNK and nuclear factor-kappa B in interleukin-1beta-induced matrix metalloproteinase-9 expression in rat brain astrocytes. J. Neurochem. 2004, 90, 1477–1488. [Google Scholar] [CrossRef]
- St-Pierre, Y.; Couillard, J.; Van Themsche, C. Regulation of MMP-9 gene expression for the development of novel molecular targets against cancer and inflammatory diseases. Expert Opin. Ther. Targets 2004, 8, 473–489. [Google Scholar] [CrossRef]
- Tseng, H.C.; Lee, I.T.; Lin, C.C.; Chi, P.L.; Cheng, S.E.; Shih, R.H.; Hsiao, L.D.; Yang, C.M. IL-1beta promotes corneal epithelial cell migration by increasing MMP-9 expression through NF-kappaB- and AP-1-dependent pathways. PLoS ONE 2013, 8, e57955. [Google Scholar] [CrossRef]
- Wu, C.Y.; Hsieh, H.L.; Sun, C.C.; Yang, C.M. IL-1beta induces MMP-9 expression via a Ca2+-dependent CaMKII/JNK/c-JUN cascade in rat brain astrocytes. Glia 2009, 57, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Lee, I.T.; Wu, W.B.; Liu, C.J.; Hsieh, H.L.; Hsiao, L.D.; Yang, C.C.; Yang, C.M. Thrombin mediates migration of rat brain astrocytes via PLC, Ca(2)(+), CaMKII, PKCalpha, and AP-1-dependent matrix metalloproteinase-9 expression. Mol. Neurobiol. 2013, 48, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Hu, Z.L.; Huang, C.; Yu, X.W.; Fan, H.; Yang, J.W.; Fang, P.; Ni, L.; Chen, J.G.; Wang, F. Orexin-A promotes cell migration in cultured rat astrocytes via Ca2+-dependent PKCalpha and ERK1/2 signals. PLoS ONE 2014, 9, e95259. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Wilhelmsson, U.; Pekna, M. The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. 2014, 565, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Huang, Y.; Tian, C.; Zhao, Y.; Zheng, J. FOXO3a inhibits TNF-alpha- and IL-1beta-induced astrocyte proliferation:Implication for reactive astrogliosis. Glia 2011, 59, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Kuo, C.T.; Lin, C.C.; Hsieh, H.L.; Yang, C.M. IL-1beta induces expression of matrix metalloproteinase-9 and cell migration via a c-Src-dependent, growth factor receptor transactivation in A549 cells. Br. J. Pharmacol. 2010, 160, 1595–1610. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Wang, H.H.; Wu, C.Y.; Tung, W.H.; Yang, C.M. Lipoteichoic acid induces matrix metalloproteinase-9 expression via transactivation of PDGF receptors and NF-kappaB activation in rat brain astrocytes. Neurotox. Res. 2010, 17, 344–359. [Google Scholar] [CrossRef]
- Lee, C.W.; Lin, C.C.; Lin, W.N.; Liang, K.C.; Luo, S.F.; Wu, C.B.; Wang, S.W.; Yang, C.M. TNF-alpha induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-kappaB/p300 binding in human tracheal smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L799–L812. [Google Scholar] [CrossRef]
- Dong, L.H.; Wen, J.K.; Miao, S.B.; Jia, Z.; Hu, H.J.; Sun, R.H.; Wu, Y.; Han, M. Baicalin inhibits PDGF-BB-stimulated vascular smooth muscle cell proliferation through suppressing PDGFRbeta-ERK signaling and increase in p27 accumulation and prevents injury-induced neointimal hyperplasia. Cell Res. 2010, 20, 1252–1262. [Google Scholar] [CrossRef]
- Yang, C.M.; Yang, S.H.; Lee, T.H.; Fang, J.Y.; Lin, C.F.; Jou, M.J.; Hsieh, H.L. Evaluation of Anti-Inflammatory Effects of Helminthostachys zeylanica Extracts via Inhibiting Bradykinin-Induced MMP-9 Expression in Brain Astrocytes. Mol. Neurobiol. 2016, 53, 5995–6005. [Google Scholar] [CrossRef]
- Wong, T.F.; Takeda, T.; Li, B.; Tsuiji, K.; Kondo, A.; Tadakawa, M.; Nagase, S.; Yaegashi, N. Curcumin targets the AKT-mTOR pathway for uterine leiomyosarcoma tumor growth suppression. Int. J. Clin. Oncol. 2014, 19, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Deeb, D.; Gao, X.; Jiang, H.; Janic, B.; Arbab, A.S.; Rojanasakul, Y.; Dulchavsky, S.A.; Gautam, S.C. Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells through a ROS-dependent mechanism. Biochem. Pharmacol. 2010, 79, 350–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.C.; Lin, C.C.; Hsiao, L.D.; Yang, C.M. Galangin Inhibits Thrombin-Induced MMP-9 Expression in SK-N-SH Cells via Protein Kinase-Dependent NF-kappaB Phosphorylation. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Bornhorst, J.; Nguyen, T.T.; Aschner, M. Oxidative stress mechanisms underlying Parkinson’s disease-associated neurodegeneration in C. elegans. Int. J. Mol. Sci. 2013, 14, 23103–23128. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Cardaioli, E.; Da Pozzo, P.; Formichi, P.; Gallus, G.N.; Radi, E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012, 322, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Pestana, R.R.; Kinjo, E.R.; Hernandes, M.S.; Britto, L.R. Reactive oxygen species generated by NADPH oxidase are involved in neurodegeneration in the pilocarpine model of temporal lobe epilepsy. Neurosci. Lett. 2010, 484, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Sesti, F. Oxidation of K(+) Channels in Aging and Neurodegeneration. Aging Dis. 2016, 7, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.U.; Shah, S.A.; Kim, M.O. Glycine inhibits ethanol-induced oxidative stress, neuroinflammation and apoptotic neurodegeneration in postnatal rat brain. Neurochem. Int. 2016, 96, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.H.; Oh, Y.C.; Cho, W.K.; Lee, B.; Ma, J.Y. Anti-Inflammatory Effects of Melandrii Herba Ethanol Extract via Inhibition of NF-kappaB and MAPK Signaling Pathways and Induction of HO-1 in RAW 264.7 Cells and Mouse Primary Macrophages. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Liu, L.; Yu, D.H.; Li, X.Z.; Zhou, Q.; Liu, S.M. Therapeutic effect of Rhizoma Dioscoreae Nipponicae on gouty arthritis based on the SDF-1/CXCR 4 and p38 MAPK pathway: An in vivo and in vitro study. Phytother Res. 2014, 28, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Meng, X.; Wang, J.; Sun, J.; Ren, X.; Qin, M.; Sun, J.; Sun, G.; Sun, X. Notoginsenoside R1 attenuates amyloid-beta-induced damage in neurons by inhibiting reactive oxygen species and modulating MAPK activation. Int. Immunopharmacol. 2014, 22, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, J.; Ye, J.; Zhai, X.; Song, J.; Jiang, C.; Wang, J.; Zhang, H.; Jia, X.; Zhu, F. Anti-inflammatory effect of the six compounds isolated from Nauclea officinalis Pierrc ex Pitard, and molecular mechanism of strictosamide via suppressing the NF-kappaB and MAPK signaling pathway in LPS-induced RAW 264.7 macrophages. J. Ethnopharmacol. 2017, 196, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Cao, J.; Fang, L.; Zhao, H.; Liu, Z.; Ran, J.; Zheng, X.; Li, X.; Zhou, Y.; Ge, D.; et al. Geniposide suppresses LPS-induced nitric oxide, PGE2 and inflammatory cytokine by downregulating NF-kappaB, MAPK and AP-1 signaling pathways in macrophages. Int. Immunopharmacol. 2014, 20, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kunworarath, N.; Rangkadilok, N.; Suriyo, T.; Thiantanawat, A.; Satayavivad, J. Longan (Dimocarpus longan Lour.) inhibits lipopolysaccharide-stimulated nitric oxide production in macrophages by suppressing NF-kappaB and AP-1 signaling pathways. J. Ethnopharmacol. 2016, 179, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Jou, T.C.; Jou, M.J.; Chen, J.Y.; Lee, S.Y. [Properties of rat brain astrocytes in long-term culture]. Taiwan Yi Xue Hui Za Zhi. J. Formos. Med Assoc. 1985, 84, 865–881. [Google Scholar]
- Lin, C.C.; Yang, C.C.; Hsiao, L.D.; Chen, S.Y.; Yang, C.M. Heme Oxygenase-1 Induction by Carbon Monoxide Releasing Molecule-3 Suppresses Interleukin-1beta-Mediated Neuroinflammation. Front. Mol. Neurosci. 2017, 10, 387. [Google Scholar] [CrossRef]
- Eberhardt, W.; Schulze, M.; Engels, C.; Klasmeier, E.; Pfeilschifter, J. Glucocorticoid-mediated suppression of cytokine-induced matrix metalloproteinase-9 expression in rat mesangial cells: Involvement of nuclear factor-kappaB and Ets transcription factors. Mol. Endocrinol. 2002, 16, 1752–1766. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Wu, C.Y.; Yang, C.M. Bradykinin induces matrix metalloproteinase-9 expression and cell migration through a PKC-delta-dependent ERK/Elk-1 pathway in astrocytes. Glia 2008, 56, 619–632. [Google Scholar] [CrossRef]
- Ding, M.; Huang, C.; Lu, Y.; Bowman, L.; Castranova, V.; Vallyathan, V. Involvement of protein kinase C in crystalline silica-induced activation of the MAP kinase and AP-1 pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L291–L297. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Guillamon, M.; Delgado, P.; Ortega, L.; Pares, M.; Rosell, A.; Garcia-Bonilla, L.; Fernandez-Cadenas, I.; Borrell-Pages, M.; Boada, M.; Montaner, J. Neuronal TIMP-1 release accompanies astrocytic MMP-9 secretion and enhances astrocyte proliferation induced by beta-amyloid 25-35 fragment. J. Neurosci. Res. 2009, 87, 2115–2125. [Google Scholar] [CrossRef]
- Leonardo, C.C.; Eakin, A.K.; Ajmo, J.M.; Collier, L.A.; Pennypacker, K.R.; Strongin, A.Y.; Gottschall, P.E. Delayed administration of a matrix metalloproteinase inhibitor limits progressive brain injury after hypoxia-ischemia in the neonatal rat. J. Neuroinflammation. 2008, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Wang, X.; Baba, M. HIV-1-infected macrophages induce astrogliosis by SDF-1alpha and matrix metalloproteinases. Biochem. Biophys. Res. Commun. 2005, 336, 1214–1220. [Google Scholar] [CrossRef] [PubMed]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-C.; Lin, C.-C.; Jou, M.-J.; Hsiao, L.-D.; Yang, C.-M. RTA 408 Inhibits Interleukin-1β-Induced MMP-9 Expression via Suppressing Protein Kinase-Dependent NF-κB and AP-1 Activation in Rat Brain Astrocytes. Int. J. Mol. Sci. 2019, 20, 2826. https://doi.org/10.3390/ijms20112826
Yang C-C, Lin C-C, Jou M-J, Hsiao L-D, Yang C-M. RTA 408 Inhibits Interleukin-1β-Induced MMP-9 Expression via Suppressing Protein Kinase-Dependent NF-κB and AP-1 Activation in Rat Brain Astrocytes. International Journal of Molecular Sciences. 2019; 20(11):2826. https://doi.org/10.3390/ijms20112826
Chicago/Turabian StyleYang, Chien-Chung, Chih-Chung Lin, Mei-Jie Jou, Li-Der Hsiao, and Chuen-Mao Yang. 2019. "RTA 408 Inhibits Interleukin-1β-Induced MMP-9 Expression via Suppressing Protein Kinase-Dependent NF-κB and AP-1 Activation in Rat Brain Astrocytes" International Journal of Molecular Sciences 20, no. 11: 2826. https://doi.org/10.3390/ijms20112826