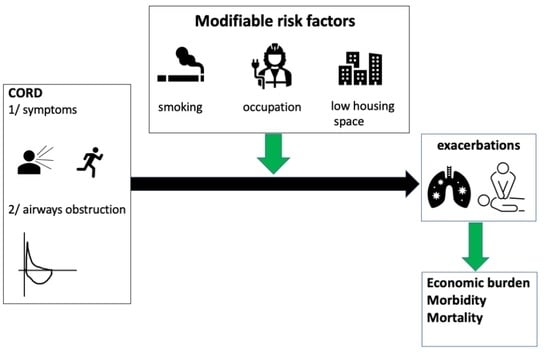
Identification of Modifiable Risk Factors of Exacerbations in Chronic Respiratory Diseases with Airways Obstruction in Vietnam
Abstract
:1. Introduction
2. Material and Methods
2.1. Population Selection
2.2. Pulmonary Function Test
2.3. Questionnaire
2.4. Definitions
2.5. Data Analysis
3. Results
3.1. Demographic and Socio-Economic Characteristics of Study Patients
3.2. Medical History and Clinical Characteristics of the Studied Population (Table 1)
3.3. Smoking Habits and Smoking Exposure of the Studied Population (Table 1)
3.4. Types of House and Indoor Pollution Factors (Table 2)
| Characteristics | n (%) | Exacerbation + n (%) | OR (95% CI) | p Value * |
|---|---|---|---|---|
| Total | 235 | 56 | ||
| Type of house | ||||
| Others | 116 (49.4) | 34 (29.3) | 1 | 0.052 |
| Tube and rent house | 119 (50.6) | 22 (18.5) | 0.55 (0.29–1.01) | |
| Housing space per person | ||||
| <10 m2 of floor/person | 93 (39.6) | 29 (31.2) | 1 | 0.032 |
| ≥10 m2 of floor/person | 142 (60.4) | 27 (19.0) | 0.52 (0.29–0.95) | |
| Extractor fan | ||||
| No | 166 (70.6) | 46 (22.7) | 1 | 0.030 |
| Yes | 69 (29.4) | 10 (14.5) | 0.44 (0.21–0.94) | |
| Air conditioner | ||||
| No | 101 (43.0) | 28 (27.7) | 1 | NS |
| Yes | 134 (57.0) | 28 (20.9) | 0.69 (0.38–1.26) | |
| Indoor incense burning | ||||
| No | 43 (18.3) | 13 (30.2) | 1 | |
| Yes | 192 (81.7) | 43 (22.4) | 0.67 (0.32–1.39) | NS |
| Using biofuel for stove | ||||
| No | 201 (85.5) | 45 (22.4) | 1 | NS |
| Yes | 34 (14.5) | 11 (32.4) | 1.66 (0.75–3.66) | |
| Using volatile compounds | ||||
| No | 149 (63.4) | 40 (26.8) | 1 | NS |
| Yes | 86 (36.6) | 16 (18.6) | 0.62 (0.32–1.20) | |
| Using insecticides | ||||
| No | 165 (70.2) | 42 (25.5) | 1 | NS |
| Yes | 70 (29.8) | 14 (20.0) | 0.73 (0.37–1.45) | |
| Childhood biofuel exposure | ||||
| No | 49 (20.9) | 8 (17.8) | 1 | NS |
| Yes | 186 (79.1) | 48 (25.3) | 1.57 (0.68–3.59) |
3.5. Risk Factors for Exacerbation Occurrence on Univariate Analysis
3.6. Risk Factors for Occurrence of Exacerbation on Multivariate Analysis (Table 3 and Figure 2)
| Adjusted OR (95% CI) | p Value * | |
|---|---|---|
| Occupational exposure | ||
| No | 1 | |
| Yes | 2.46 (1.17–5.21) | 0.018 |
| Housing space per person | ||
| ≤10 m2 | 1 | 0.046 |
| >10 m2 | 0.53 (0.28–0.99) |

4. Discussion
4.1. Smoking
4.2. Occupational Exposure
4.3. Indoor Air Pollution
4.4. Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Labaki, W.W.; Han, M.K. Chronic respiratory diseases: A global view. Lancet Respir. Med. 2020, 8, 531–533. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Prevention, Diagnosis and Management of COPD: 2022 Report. 2022. Available online: https://goldcopd.org/2022-gold-reports-2/ (accessed on 7 April 2022).
- Viniol, C.; Vogelmeier, C.F. Exacerbations of COPD. Eur. Respir. Rev. 2018, 27, 170103. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, M.T.; Kunisaki, K.M.; Strand, M.J.; Anzueto, A.; Bhatt, S.P.; Bowler, R.P.; Criner, G.J.; Curtis, J.L.; Hanania, N.A.; Nath, H.; et al. Acute Exacerbations and Lung Function Loss in Smokers with and without Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 195, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Soremekun, S.; Heaney, L.G.; Skinner, D.; Bulathsinhala, L.; Carter, V.; Chaudhry, I.; Hosseini, N.; Eleangovan, N.; Murray, R.; Tran, T.N.; et al. Asthma exacerbations are associated with a decline in lung function: A longitudinal population-based study. Thorax 2022. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Preventing Chronic Diseases: A Vital Investment: WHO Global Report; World Health Organization: Geneva, Switzerland, 2012; Available online: https://apps.who.int/iris/bitstream/handle/10665/43314/9241563001_eng.pdf?sequence=1&isAllowed=y (accessed on 7 April 2022).
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Agnew, M. Spirometry in clinical use: Practical issues. Breathe 2010, 6, 196–203. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2021. 2021. Available online: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf (accessed on 7 April 2022).
- Willemse, B.W.M.; Postma, D.S.; Timens, W.; ten Hacken, N.H.T. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur. Respir. J. 2004, 23, 464–476. [Google Scholar] [CrossRef]
- Riesco-Miranda, J.A.; Alcazar-Navarrete, B.; Carrero, J.A.T.; Campuzano, A.; Pérez, J.; Lorenzo, J.L. Active smoking and COPD phenotype: Distribution and impact on prognostic factors. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1989–1999. [Google Scholar] [CrossRef]
- Bittner, J.; Hasegawa, K.; Probst, B.D.; Mould-Millman, N.-K.; Silverman, R.A.; Camargo, C.A., Jr. Smoking status and smoking cessation intervention among U.S. adults hospitalized for asthma exacerbation. Allergy Asthma Proc. 2016, 37, 318–323. [Google Scholar] [CrossRef]
- Lange, P.; Groth, S.; Nyboe, G.J.; Mortensen, J.; Appleyard, M.; Jensen, G.; Schnohr, P. Effects of smoking and changes in smoking habits on the decline of FEV1. Eur. Respir. J. 1989, 2, 811–816. [Google Scholar] [PubMed]
- Tommola, M.; Ilmarinen, P.; Tuomisto, L.E.; Haanpää, J.; Kankaanranta, T.; Niemelä, O.; Kankaanranta, H. The effect of smoking on lung function: A clinical study of adult-onset asthma. Eur. Respir. J. 2016, 48, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Coultas, D.B. Health effects of passive smoking. 8. Passive smoking and risk of adult asthma and COPD: An update. Thorax 1998, 53, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Van Minh, H.; Giang, K.B.; Ngoc, N.B.; Hai, P.T.; Huyen, D.T.T.; Khue, L.N.; Lam, N.T.; Nga, P.T.Q.; Quan, N.T.; Xuyen, N.T. Prevalence of tobacco smoking in Vietnam: Findings from the Global Adult Tobacco Survey 2015. Int. J. Public Health 2017, 62, 121–129. [Google Scholar] [CrossRef]
- Eisner, M.D.; Anthonisen, N.; Coultas, D.; Kuenzli, N.; Perez-Padilla, R.; Postma, D.; Romieu, I.; Silverman, E.K.; Balmes, J.R.; Environmental and Occupational Health Assembly. An Official American Thoracic Society Public Policy Statement: Novel Risk Factors and the Global Burden of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2010, 182, 693–718. [Google Scholar] [CrossRef]
- Kim, J.-L.; Henneberger, P.K.; Lohman, S.; Olin, A.-C.; Dahlman-Höglund, A.; Andersson, E.; Torén, K.; Holm, M. Impact of occupational exposures on exacerbation of asthma: A population-based asthma cohort study. BMC Pulm. Med. 2016, 16, 148. [Google Scholar] [CrossRef]
- Nishida, C.; Yatera, K. The Impact of Ambient Environmental and Occupational Pollution on Respiratory Diseases. Int. J. Environ. Res. Public Health 2022, 19, 2788. [Google Scholar] [CrossRef]
- Dement, J.M.; Cloeren, M.; Ringen, K.; Quinn, P.; Chen, A.; Cranford, K.; Haas, S.; Hines, S. COPD risk among older construction workers—Updated analyses 2020. Am. J. Ind. Med. 2021, 64, 462–475. [Google Scholar] [CrossRef]
- Stoleski, S.; Minov, J.; Karadzinska-Bislimovska, J.; Mijakoski, D.; Atanasovska, A.; Bislimovska, D. Asthma and Chronic Obstructive Pulmonary Disease Associated with Occupational Exposure in Dairy Farmers—Importance of Job Exposure Matrices. Open Access Maced. J. Med. Sci. 2019, 7, 2350–2359. [Google Scholar] [CrossRef]
- Torén, K.; Järvholm, B. Effect of Occupational Exposure to Vapors, Gases, Dusts, and Fumes on COPD Mortality Risk among Swedish Construction Workers: A longitudinal cohort study. Chest 2014, 145, 992–997. [Google Scholar] [CrossRef]
- de Jong, K.; Boezen, H.M.; Kromhout, H.; Vermeulen, R.; Postma, D.S.; Vonk, J.M. LifeLines Cohort study Pesticides and other occupational exposures are associated with airway obstruction: The LifeLines cohort study. Occup. Environ. Med. 2014, 71, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Toren, K.; Vikgren, J.; Olin, A.-C.; Rosengren, A.; Bergström, G.; Brandberg, J. Occupational exposure to vapor, gas, dust, or fumes and chronic airflow limitation, COPD, and emphysema: The Swedish CArdioPulmonary BioImage Study (SCAPIS pilot). Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 3407–3413. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.J.; Miedinger, D.; Keidel, D.; Bettschart, R.; Bircher, A.; Bridevaux, P.-O.; Curjuric, I.; Kromhout, H.; Rochat, T.; Rothe, T.; et al. Occupational Exposure to Dusts, Gases, and Fumes and Incidence of Chronic Obstructive Pulmonary Disease in the Swiss Cohort Study on Air Pollution and Lung and Heart Diseases in Adults. Am. J. Respir. Crit. Care Med. 2012, 185, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Sunwoo, Y.E.; Lee, S.-Y.; Lee, C.-K.; Kim, J.-H.; Lee, J.-T.; Kim, D.-H. Chronic Obstructive Pulmonary Disease (COPD) and Vapors, Gases, Dusts, or Fumes (VGDF): A Meta-analysis. COPD J. Chronic Obstr. Pulm. Dis. 2014, 12, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Jaén, Á.; Zock, J.P.; Kogevinas, M.; Ferrer, A.; Marín, A. Occupation, smoking, and chronic obstructive respiratory disorders: A cross sectional study in an industrial area of Catalonia, Spain. Environ. Health 2006, 5, 2. [Google Scholar] [CrossRef]
- Boschetto, P.; Quintavalle, S.; Miotto, D.; Cascio, N.L.; Zeni, E.; Mapp, C.E. Chronic obstructive pulmonary disease (COPD) and occupational exposures. J. Occup. Med. Toxicol. 2006, 1, 11. [Google Scholar] [CrossRef]
- Blanc, P.D.; Annesi-Maesano, I.; Balmes, J.R.; Cummings, K.J.; Fishwick, D.; Miedinger, D.; Murgia, N.; Naidoo, R.N.; Reynolds, C.J.; Sigsgaard, T.; et al. The Occupational Burden of Nonmalignant Respiratory Diseases. An Official American Thoracic Society and European Respiratory Society Statement. Am. J. Respir. Crit. Care Med. 2019, 199, 1312–1334. [Google Scholar] [CrossRef]
- Pallasaho, P.; Kainu, A.; Sovijärvi, A.; Lindqvist, A.; Piirilä, P. Combined Effect of Smoking and Occupational Exposure to Dusts, Gases or Fumes on the Incidence of COPD. COPD J. Chronic Obstr. Pulm. Dis. 2013, 11, 88–95. [Google Scholar] [CrossRef]
- Blanc, P.D.; Iribarren, C.; Trupin, L.; Earnest, G.; Katz, P.P.; Balmes, J.; Sidney, S.; Eisner, M.D. Occupational exposures and the risk of COPD: Dusty trades revisited. Thorax 2009, 64, 6–12. [Google Scholar] [CrossRef]
- Grahn, K.; Gustavsson, P.; Andersson, T.; Lindén, A.; Hemmingsson, T.; Selander, J.; Wiebert, P. Occupational exposure to particles and increased risk of developing chronic obstructive pulmonary disease (COPD): A population-based cohort study in Stockholm, Sweden. Environ. Res. 2021, 200, 111739. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Di-Oxide, Sulfur Dioxide and Carbon Monoxide 2021. Available online: https://apps.who.int/iris/handle/10665/345329 (accessed on 1 July 2022).
- Van Tran, V.; Park, D.; Lee, Y.-C. Indoor Air Pollution, Related Human Diseases, and Recent Trends in the Control and Improvement of Indoor Air Quality. Int. J. Environ. Res. Public Health 2020, 17, 2927. [Google Scholar] [CrossRef] [PubMed]
- Mark Crosby, T.D. Space Standards for Homes; Royal Institute of British Architects (RIBA): London, UK, 2015; Available online: https://www.architecture.com/knowledge-and-resources/resources-landing-page/space-standards-for-homes#available-resources (accessed on 1 July 2022).
- Ministry of Construction in Vietnam. Average Housing Floor Area per Person in Vietnam 2021. Available online: https://baotainguyenmoitruong.vn/phan-dau-dien-tich-nha-o-binh-quan-dau-nguoi-dat-27m2-vao-nam-2030-332509.html (accessed on 1 July 2022).
- Bu-Olayan, A.H.; Thomas, B.V. Exposition of respiratory ailments from trace metals concentrations in incenses. Sci. Rep. 2021, 11, 10210. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Ho, W.-C.; Yu, Y.-H. Adolescent lung function associated with incense burning and other environmental exposures at home. Indoor Air 2017, 27, 746–752. [Google Scholar] [CrossRef]
- Guo, S.-E.; Chi, M.-C.; Lin, C.-M.; Yang, T.-M. Contributions of burning incense on indoor air pollution levels and on the health status of patients with chronic obstructive pulmonary disease. PeerJ 2020, 8, e9768. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Peng, H.-C.; Shih, J.-Y.; Yu, C.-J.; Yang, P.-C.; Chen, Y.-Y. Association between air pollution and exacerbations of chronic obstructive pulmonary disease and asthma in adult citizens of Yunlin County in Taiwan. Eur. Respir. J. 2017, 50 (Suppl. S61), PA2635. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, J.; Zhang, H.; Shi, C.; Li, G.; Peng, Z.; Ma, J.; Zhou, Y.; Zhang, L. Short-Term Exposure to Ambient Air Pollution and Asthma Mortality. Am. J. Respir. Crit. Care Med. 2019, 200, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Shetty, B.S.P.; D’Souza, G.; Padukudru Anand, M. Effect of Indoor Air Pollution on Chronic Obstructive Pulmonary Disease (COPD) Deaths in Southern Asia-A Systematic Review and Meta-Analysis. Toxics 2021, 9, 85. [Google Scholar] [CrossRef]
- Fernandes, A.G.O.; De Souza-Machado, C.; Pinheiro, G.P.; De Oliva, S.T.; Mota, R.C.L.; De Lima, V.B.; Cruz, C.S.; Chatkin, J.M.; Cruz, A. Dual exposure to smoking and household air pollution is associated with an increased risk of severe asthma in adults in Brazil. Clin. Transl. Allergy 2018, 8, 48. [Google Scholar] [CrossRef]
- Balmes, J.R. Household air pollution from domestic combustion of solid fuels and health. J. Allergy Clin. Immunol. 2019, 143, 1979–1987. [Google Scholar] [CrossRef]
- Cho, J.; on behalf of the KOLD and KOCOSS Investigators; Lee, C.-H.; Hwang, S.-S.; Kim, K.U.; Lee, S.H.; Park, H.Y.; Park, S.J.; Min, K.H.; Oh, Y.-M.; et al. Risk of acute exacerbations in chronic obstructive pulmonary disease associated with biomass smoke compared with tobacco smoke. BMC Pulm. Med. 2019, 19, 68. [Google Scholar] [CrossRef]

| Characteristics | n (%) | Exacerbation + n (%) | OR (95% CI) | p Value * |
|---|---|---|---|---|
| Total | 235 | 56 | ||
| Sex | ||||
| Female | 60 (25.5) | 13 (21.7) | 1 | NS |
| Male | 175 (74.5) | 43 (24.6) | 1.18 (0.58–2.38) | |
| Education level | ||||
| Primary | 127 (54.0) | 38 (29.9) | 1 | 0.017 |
| Secondary/Post-secondary | 108 (46.0) | 18 (16.7) | 0.47 (0.25–0.88) | |
| Occupation | ||||
| Non-exposed | 81 (34.5) | 11 (13.6) | 1 | 0.007 |
| Exposed | 154 (65.5) | 45 (29.2) | 2.63 (1.27–5.42) | |
| History of tuberculosis | ||||
| No | 203 (86.4) | 45 (22.2) | 1 | NS |
| Yes | 32 (13.6) | 11 (34.4) | 1.84 (0.82–4.10) | |
| History of allergy | ||||
| No | 140 (59.6) | 32 (22.9) | 1 | NS |
| Yes | 95 (40.4) | 24 (25.3) | 1.14 (0.62–2.10) | |
| Clinical diagnosis | ||||
| Asthma | 104 (44.3) | 19 (18.3) | 1 | NS |
| COPD | 131 (55.7) | 37 (28.2) | 1.76 (0.94–3.29) | |
| mMRC grade | ||||
| grade 1 | 111 (47.2) | 17 (15.3) | 1 | 0.005 |
| grade ≥ 2 | 124 (52.8) | 39 (31.5) | 2.48 (1.31–4.71) | |
| FEV1 post-BD | ||||
| <60% PV | 128 (54.5) | 37 (28.9) | 1 | 0.046 |
| ≥60% PV | 107 (45.5) | 19 (17.8) | 0.53 (0.28–0.99) | |
| Smoking status | ||||
| No | 73 (31.2) | 12 (16.4) | 1 | 0.074 |
| Yes | 162 (68.8) | 44 (27.2) | 1.90 (0.93–3.85) | |
| Pack years ≥ 20 | ||||
| No | 111 (47.2) | 20 (18.0) | 1 | 0.048 |
| Yes | 124 (52.8) | 36 (29.0) | 1.86 (1.01–3.46) | |
| Exposure to smoke from parents in childhood | ||||
| No | 128 (54.5) | 26 (21.5) | 1 | NS |
| Yes | 107 (45.5) | 30 (26.3) | 1.30 (0.71–2.38) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.C.; Tran, H.V.T.; Nguyen, T.H.; Vo, D.C.; Godin, I.; Michel, O. Identification of Modifiable Risk Factors of Exacerbations in Chronic Respiratory Diseases with Airways Obstruction in Vietnam. Int. J. Environ. Res. Public Health 2022, 19, 11088. https://doi.org/10.3390/ijerph191711088
Nguyen TC, Tran HVT, Nguyen TH, Vo DC, Godin I, Michel O. Identification of Modifiable Risk Factors of Exacerbations in Chronic Respiratory Diseases with Airways Obstruction in Vietnam. International Journal of Environmental Research and Public Health. 2022; 19(17):11088. https://doi.org/10.3390/ijerph191711088
Chicago/Turabian StyleNguyen, Thuy Chau, Hoa Vi T. Tran, Thanh Hiep Nguyen, Duc Chien Vo, Isabelle Godin, and Olivier Michel. 2022. "Identification of Modifiable Risk Factors of Exacerbations in Chronic Respiratory Diseases with Airways Obstruction in Vietnam" International Journal of Environmental Research and Public Health 19, no. 17: 11088. https://doi.org/10.3390/ijerph191711088