Carbamazepine and Diclofenac Removal Double Treatment: Oxidation and Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Analytical Method
2.3. Adsorption Tests
2.4. Oxidation Tests
2.5. Combined Tests
3. Results and Discussion
3.1. Activated Carbon
3.2. Carbamazepine and Diclofenac Adsorption Tests
3.3. Carbamazepine and Diclofenac Oxidation Tests
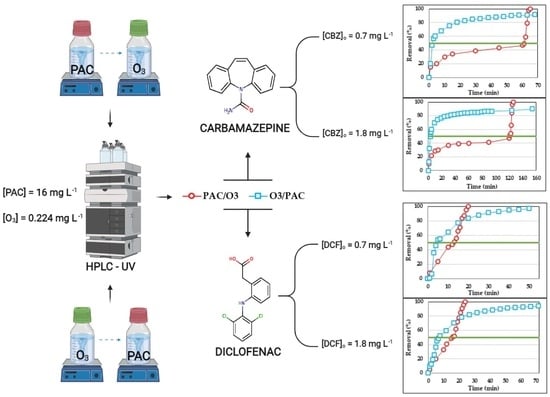
3.4. Combined Tests for Carbamazepine and Diclofenac PAC/O3 and O3/PAC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Deng, W.; Li, N.; Zheng, H.; Lin, H. Occurrence and risk assessment of antibiotics in river water in Hong Kong. Ecotoxicol. Environ. Saf. 2016, 125, 121–127. [Google Scholar] [CrossRef]
- Nazari, G.; Abolghasemi, H.; Esmaieli, M. Batch adsorption of cephalexin antibiotic from aqueous solution by walnut shell-based activated carbon. J. Taiwan Inst. Chem. Eng. 2016, 58, 357–365. [Google Scholar] [CrossRef]
- Carmona, E.; Andreu, V.; Picó, Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water. Sci. Total Environ. 2014, 484, 53–63. [Google Scholar] [CrossRef]
- Yuan, X.; Qiang, Z.; Ben, W.; Zhu, B.; Liu, J. Rapid detection of multiple class pharmaceuticals in both municipal wastewater and sludge with ultra high performance liquid chromatography tandem mass spectrometry. J. Environ. Sci. 2014, 26, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Nevitt, S.J.; Marson, A.G.; Smith, C.T. Carbamazepine versus phenytoin monotherapy for epilepsy: An individual participant data review. Cochrane Database Syst. Rev. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Powell, G.; Saunders, M.; Rigby, A.; Marson, A.G. Immediate-release versus controlled-release carbamazepine in the treatment of epilepsy. Cochrane Database Syst. Rev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Lindenberg, M.; Kopp, S.; Dressman, J.B. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur. J. Pharm. Biopharm. 2004, 58, 265–278. [Google Scholar] [CrossRef]
- NCBI, 2021 National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 20 May 2021).
- Ferrari, B.; Paxéus, N.; Lo Giudice, R.; Pollio, A.; Garric, J. Ecotoxicological impact of pharmaceuticals found in treated wastewaters: Study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicol. Environ. Saf. 2003, 55, 359–370. [Google Scholar] [CrossRef]
- van den Brandhof, E.J.; Montforts, M. Fish embryo toxicity of carbamazepine, diclofenac and metoprolol. Ecotoxicol. Environ. Saf. 2010, 73, 1862–1866. [Google Scholar] [CrossRef]
- Han, G.H.; Hur, H.G.; Kim, S.D. Ecotoxicological risk of pharmaceuticals from wastewater treatment plants in Korea: Occurrence and toxicity to Daphnia magna. Environ. Toxicol. Chem. 2006, 25, 265–271. [Google Scholar] [CrossRef]
- Nunes, M.; Fischer, L.W.; do Valle Freitas, R.D.; Miraglia, S.M.; Oliva, S.U. Efeitos da carbamazepina sobre o epidídimo e possíveis impactos sobre a maturação espermática. Braz. J. Health Rev. 2020, 3, 4091–4101. [Google Scholar] [CrossRef]
- Jentink, J.; Dolk, H.; Loane, M.A.; Morris, J.K.; Wellesley, D.; Garne, E.; De Jong-van Den Berg, L. Intrauterine exposure to carbamazepine and specific congenital malformations: Systematic review and case-control study. BMJ 2010, 341, 1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, C.; Stewart, M.; Stevenson, M.; Morrow, J.; Nelson, J. Neurodevelopment of children exposed in utero to lamotrigine, sodium valproate and carbamazepine. Arch. Dis. Child. 2011, 96, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, D.E.; Brice-Bennett, S.; D’Souza, S.W. Antiepileptic medication during pregnancy: Does fetal genotype affect outcome? Pediatr. Res. 2007, 62, 120–127. [Google Scholar] [CrossRef]
- European Parliament Directive 2000/60/EC. Establishing a framework for Community action in the field of water policy. D. Communities Eur. 2000, 327, 1–73. [Google Scholar]
- Al Aukidy, M.; Verlicchi, P.; Jelic, A.; Petrovic, M.; Barcelò, D. Monitoring release of pharmaceutical compounds: Occurrence and environmental risk assessment of two WWTP effluents and their receiving bodies in the Po Valley, Italy. Sci. Total Environ. 2012, 438, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gin, K.Y.H.; Lin, A.Y.C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef]
- Singer, H.; Jaus, S.; Hanke, I.; Lück, A.; Hollender, J.; Alder, A.C. Determination of biocides and pesticides by on-line solid phase extraction coupled with mass spectrometry and their behaviour in wastewater and surface water. Environ. Pollut. 2010, 158, 3054–3064. [Google Scholar] [CrossRef]
- Christoffels, E.; Brunsch, A.; Wunderlich-Pfeiffer, J.; Mertens, F.M. Monitoring micropollutants in the Swist river basin. Water Sci. Technol. 2016, 74, 2280–2296. [Google Scholar] [CrossRef] [PubMed]
- Kinney, C.A.; Furlong, E.T.; Werner, S.L.; Cahill, J.D. Presence and distribution of wastewater-derived pharmaceuticals in soil irrigated with reclaimed water. Environ. Toxicol. Chem. 2006, 25, 317–326. [Google Scholar] [CrossRef]
- Weigel, S.; Berger, U.; Jensen, E.; Kallenborn, R.; Thoresen, H.; Hühnerfuss, H. Determination of selected pharmaceuticals and caffeine in sewage and seawater from Tromsø/Norway with emphasis on ibuprofen and its metabolites. Chemosphere 2004, 56, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Weigel, S.; Bester, K.; Hühnerfuss, H. New method for rapid solid-phase extraction of large-volume water samples and its application to non-target screening of North Sea water for organic contaminants by gas chromatography-mass spectrometry. J. Chromatogr. A 2001, 912, 151–161. [Google Scholar] [CrossRef]
- Strauch, G.; Möder, M.; Wennrich, R.; Osenbrück, K.; Gläser, H.R.; Schladitz, T.; Müller, C.; Schirmer, K.; Reinstorf, F.; Schirmer, M. Indicators for assessing anthropogenic impact on urban surface and groundwater. J. Soils Sediments 2008, 8, 23–33. [Google Scholar] [CrossRef]
- Wolf, L.; Zwiener, C.; Zemann, M. Tracking artificial sweeteners and pharmaceuticals introduced into urban groundwater by leaking sewer networks. Sci. Total Environ. 2012, 430, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Murakami, M.; Oguma, K.; Muramatsu, Y.; Takada, H.; Takizawa, S. Assessment of groundwater pollution in Tokyo using PPCPs as sewage markers. Environ. Sci. Technol. 2012, 46, 1455–1464. [Google Scholar] [CrossRef]
- Reh, R.; Licha, T.; Geyer, T.; Nödler, K.; Sauter, M. Occurrence and spatial distribution of organic micro-pollutants in a complex hydrogeological karst system during low flow and high flow periods, results of a two-year study. Sci. Total Environ. 2013, 443, 438–445. [Google Scholar] [CrossRef]
- Chefetz, B.; Mualem, T.; Ben-Ari, J. Sorption and mobility of pharmaceutical compounds in soil irrigated with reclaimed wastewater. Chemosphere 2008, 73, 1335–1343. [Google Scholar] [CrossRef]
- Christou, A.; Agüera, A.; Bayona, J.M.; Cytryn, E.; Fotopoulos, V.; Lambropoulou, D.; Manaia, C.M.; Michael, C.; Revitt, M.; Schröder, P.; et al. The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment: The knowns and unknowns of the fate of antibiotics and antibiotic resistant bacteria and resistance genes—A review. Water Res. 2017, 123, 448–467. [Google Scholar] [CrossRef] [Green Version]
- Alidina, M.; Hoppe-Jones, C.; Yoon, M.; Hamadeh, A.F.; Li, D.; Drewes, J.E. The occurrence of emerging trace organic chemicals in wastewater effluents in Saudi Arabia. Sci. Total Environ. 2014, 478, 152–162. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Fernandez-Fontaina, E.; Carballa, M.; Omil, F.; Lema, J.M. Modelling cometabolic biotransformation of organic micropollutants in nitrifying reactors. Water Res. 2014, 65, 371–383. [Google Scholar] [CrossRef]
- Tran, N.H.; Urase, T.; Ngo, H.H.; Hu, J.; Ong, S.L. Insight into metabolic and cometabolic activities of autotrophic and heterotrophic microorganisms in the biodegradation of emerging trace organic contaminants. Bioresour. Technol. 2013, 146, 721–731. [Google Scholar] [CrossRef]
- Kimura, K.; Hara, H.; Watanabe, Y. Elimination of selected acidic pharmaceuticals from municipal wastewater by an activated sludge system and membrane bioreactors. Environ. Sci. Technol. 2007, 41, 3708–3714. [Google Scholar] [CrossRef] [PubMed]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Keller, E.; Alder, A.C.; Göbel, A.; McArdell, C.S.; Ternes, T.; Siegrist, H. Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res. 2005, 39, 3139–3152. [Google Scholar] [CrossRef] [PubMed]
- Tadkaew, N.; Hai, F.I.; McDonald, J.A.; Khan, S.J.; Nghiem, L.D. Removal of trace organics by MBR treatment: The role of molecular properties. Water Res. 2011, 45, 2439–2451. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Hai, F.I.; Kang, J.; Price, W.E.; Guo, W.; Ngo, H.H.; Yamamoto, K.; Nghiem, L.D. Effects of salinity build-up on biomass characteristics and trace organic chemical removal: Implications on the development of high retention membrane bioreactors. Bioresour. Technol. 2015, 177, 274–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Geißen, S.U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Wijekoon, K.C.; Hai, F.I.; Kang, J.; Price, W.E.; Guo, W.; Ngo, H.H.; Nghiem, L.D. The fate of pharmaceuticals, steroid hormones, phytoestrogens, UV-filters and pesticides during MBR treatment. Bioresour. Technol. 2013, 144, 247–254. [Google Scholar] [CrossRef]
- Vogna, D.; Marotta, R.; Andreozzi, R.; Napolitano, A.; D’Ischia, M. Kinetic and chemical assessment of the UV/H2O2 treatment of antiepileptic drug carbamazepine. Chemosphere 2004, 54, 497–505. [Google Scholar] [CrossRef]
- Alvarino, T.; Suarez, S.; Lema, J.M.; Omil, F. Understanding the removal mechanisms of PPCPs and the influence of main technological parameters in anaerobic UASB and aerobic CAS reactors. J. Hazard. Mater. 2014, 278, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Jaimes, J.A.; Postigo, C.; Melgoza-Alemán, R.M.; Aceña, J.; Barceló, D.; López de Alda, M. Study of pharmaceuticals in surface and wastewater from Cuernavaca, Morelos, Mexico: Occurrence and environmental risk assessment. Sci. Total Environ. 2018, 613–614, 1263–1274. [Google Scholar] [CrossRef]
- Dai, C.; Geissen, S.U.; Zhang, Y.; Zhang, Y.; Zhou, X. Performance evaluation and application of molecularly imprinted polymer for separation of carbamazepine in aqueous solution. J. Hazard. Mater. 2010, 184, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Krajišnik, D.; Daković, A.; Malenović, A.; Milojević-Rakić, M.; Dondur, V.; Radulović, Ž.; Milić, J. Investigation of adsorption and release of diclofenac sodium by modified zeolites composites. Appl. Clay Sci. 2013, 83–84, 322–326. [Google Scholar] [CrossRef]
- Rodríguez-Álvarez, T.; Rodil, R.; Quintana, J.B.; Triñanes, S.; Cela, R. Oxidation of non-steroidal anti-inflammatory drugs with aqueous permanganate. Water Res. 2013, 47, 3220–3230. [Google Scholar] [CrossRef]
- Wu, C.; Huang, X.; Witter, J.D.; Spongberg, A.L.; Wang, K.; Wang, D.; Liu, J. Occurrence of pharmaceuticals and personal care products and associated environmental risks in the central and lower Yangtze river, China. Ecotoxicol. Environ. Saf. 2014, 106, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Kim, M.; Shah, A.; Alaee, M.; Smyth, S.A. Occurrence and fate of antibiotic, analgesic/anti-inflammatory, and antifungal compounds in five wastewater treatment processes. Sci. Total Environ. 2014, 473–474, 235–243. [Google Scholar] [CrossRef]
- Lindberg, R.H.; Östman, M.; Olofsson, U.; Grabic, R.; Fick, J. Occurrence and behaviour of 105 active pharmaceutical ingredients in sewage waters of a municipal sewer collection system. Water Res. 2014, 58, 221–229. [Google Scholar] [CrossRef]
- Hartmann, J.; Bartels, P.; Mau, U.; Witter, M.; Tümpling, W.V.; Hofmann, J.; Nietzschmann, E. Degradation of the drug diclofenac in water by sonolysis in presence of catalysts. Chemosphere 2008, 70, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Q.; Zhou, Z.; Sharma, V.K. Occurrence, transportation, monitoring and treatment of emerging micro-pollutants in waste water—A review from global views. Microchem. J. 2013, 110, 292–300. [Google Scholar] [CrossRef]
- Falås, P.; Longrée, P.; La Cour Jansen, J.; Siegrist, H.; Hollender, J.; Joss, A. Micropollutant removal by attached and suspended growth in a hybrid biofilm-activated sludge process. Water Res. 2013, 47, 4498–4506. [Google Scholar] [CrossRef]
- Petrie, B.; McAdam, E.J.; Scrimshaw, M.D.; Lester, J.N.; Cartmell, E. Fate of drugs during wastewater treatment. TrAC Trends Anal. Chem. 2013, 49, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Kosjek, T.; Heath, E.; Pérez, S.; Petrović, M.; Barceló, D. Metabolism studies of diclofenac and clofibric acid in activated sludge bioreactors using liquid chromatography with quadrupole—Time-of-Flight mass spectrometry. J. Hydrol. 2009, 372, 109–117. [Google Scholar] [CrossRef]
- Monsalvo, V.M.; McDonald, J.A.; Khan, S.J.; Le-Clech, P. Removal of trace organics by anaerobic membrane bioreactors. Water Res. 2014, 49, 103–112. [Google Scholar] [CrossRef]
- Behera, S.K.; Kim, H.W.; Oh, J.E.; Park, H.S. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Muñoz, M.D.; Santos, J.L.; Aparicio, I.; Alonso, E. Presence of pharmaceutically active compounds in Doñana Park (Spain) main watersheds. J. Hazard. Mater. 2010, 177, 1159–1162. [Google Scholar] [CrossRef]
- Patrolecco, L.; Capri, S.; Ademollo, N. Occurrence of selected pharmaceuticals in the principal sewage treatment plants in Rome (Italy) and in the receiving surface waters. Environ. Sci. Pollut. Res. 2015, 22, 5864–5876. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Degradation of diclofenac sodium using combined processes based on hydrodynamic cavitation and heterogeneous photocatalysis. Ultrason. Sonochem. 2014, 21, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, G.; Liu, H.; Wang, Y.; He, Z.; Wang, G. Diclofenac photodegradation under simulated sunlight: Effect of different forms of nitrogen and Kinetics. J. Hazard. Mater. 2011, 192, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.L.; Zhang, Z.L.; Banks, E.; Grover, D.; Jiang, J.Q. Pharmaceutical residues in wastewater treatment works effluents and their impact on receiving river water. J. Hazard. Mater. 2009, 166, 655–661. [Google Scholar] [CrossRef]
- Wen, Z.H.; Chen, L.; Meng, X.Z.; Duan, Y.P.; Zhang, Z.S.; Zeng, E.Y. Occurrence and human health risk of wastewater-derived pharmaceuticals in a drinking water source for Shanghai, East China. Sci. Total Environ. 2014, 490, 987–993. [Google Scholar] [CrossRef]
- Bonvin, F.; Jost, L.; Randin, L.; Bonvin, E.; Kohn, T. Super-fine powdered activated carbon (SPAC) for efficient removal of micropollutants from wastewater treatment plant effluent. Water Res. 2016, 90, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, M. Removal of Pesticides from Water Using Granular Activated Carbon and Ultrafiltration Membrane—A Pilot Plant Study. J. Encapsulation Adsorpt. Sci. 2013, 03, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Altmann, J.; Sperlich, A.; Jekel, M. Integrating organic micropollutant removal into tertiary filtration: Combining PAC adsorption with advanced phosphorus removal. Water Res. 2015, 84, 58–65. [Google Scholar] [CrossRef]
- Mailler, R.; Gaspéri, J.; Coquet, Y.; Derome, C.; Buleté, A.; Vulliet, E.; Bressy, A.; Varrault, G.; Chebbo, G.; Rocher, V. Élimination des polluants émergents dans les rejets de STEP. Tech. Sci. Méthodes 2016, 28–40. [Google Scholar] [CrossRef]
- Kårelid, V.; Larsson, G.; Björlenius, B. Pilot-scale removal of pharmaceuticals in municipal wastewater: Comparison of granular and powdered activated carbon treatment at three wastewater treatment plants. J. Environ. Manag. 2017, 193, 491–502. [Google Scholar] [CrossRef]
- Skouteris, G.; Saroj, D.; Melidis, P.; Hai, F.I.; Ouki, S. The effect of activated carbon addition on membrane bioreactor processes for wastewater treatment and reclamation—A critical review. Bioresour. Technol. 2015, 185, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestre, A.S.; Bexiga, A.S.; Proença, M.; Andrade, M.; Pinto, M.L.; Matos, I.; Fonseca, I.M.; Carvalho, A.P. Activated carbons from sisal waste by chemical activation with K 2CO 3: Kinetics of paracetamol and ibuprofen removal from aqueous solution. Bioresour. Technol. 2011, 102, 8253–8260. [Google Scholar] [CrossRef]
- Nam, S.W.; Jung, C.; Li, H.; Yu, M.; Flora, J.R.V.; Boateng, L.K.; Her, N.; Zoh, K.D.; Yoon, Y. Adsorption characteristics of diclofenac and sulfamethoxazole to graphene oxide in aqueous solution. Chemosphere 2015, 136, 20–26. [Google Scholar] [CrossRef]
- Qu, J.; Wang, H.; Wang, K.; Yu, G.; Ke, B.; Yu, H.Q.; Ren, H.; Zheng, X.; Li, J.; Li, W.W.; et al. Municipal wastewater treatment in China: Development history and future perspectives. Front. Environ. Sci. Eng. 2019, 13, 1–7. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater treatment by advanced oxidation process and their worldwide research trends. Int. J. Environ. Res. Public Health 2020, 17, 170. [Google Scholar] [CrossRef] [Green Version]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A.; Li Puma, G. Treatment of winery wastewater by ozone-based advanced oxidation processes (O3, O3/UV and O3/UV/H2O2) in a pilot-scale bubble column reactor and process economics. Sep. Purif. Technol. 2010, 72, 235–241. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- Lee, Y.; Imminger, S.; Czekalski, N.; von Gunten, U.; Hammes, F. Inactivation efficiency of Escherichia coli and autochthonous bacteria during ozonation of municipal wastewater effluents quantified with flow cytometry and adenosine tri-phosphate analyses. Water Res. 2016, 101, 617–627. [Google Scholar] [CrossRef]
- Zhu, S.; Dong, B.; Gao, N.; Jiang, J. Removal of IBMP using ozonation: Role of ozone and hydroxyl radical. Desalin. Water Treat. 2016, 57, 28776–28783. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S.K.; Price, W.E. Degradation and Fate of Pharmaceutically Active Contaminants by Advanced Oxidation Processes. Curr. Pollut. Rep. 2017, 3, 268–280. [Google Scholar] [CrossRef]
- Chedeville, O.; Di Giusto, A.; Delpeux, S.; Cagnon, B. Oxidation of pharmaceutical compounds by ozonation and ozone/activated carbon coupling: A kinetic approach. Desalin. Water Treat. 2016, 57, 18956–18963. [Google Scholar] [CrossRef]
- Nebout, P.; Cagnon, B.; Delpeux, S.; Di Giusto, A.; Chedeville, O. Comparison of the efficiency of adsorption, ozonation, and ozone/activated carbon coupling for the removal of pharmaceuticals from water. J. Environ. Eng. 2016, 142. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Pocostales, P.; Alvarez, P.; Oropesa, A. Diclofenac removal from water with ozone and activated carbon. J. Hazard. Mater. 2009, 163, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Rozas, O.; Baeza, C.; Núñez, K.; Rossner, A.; Urrutia, R.; Mansilla, H.D. Organic micropollutants (OMPs) oxidation by ozone: Effect of activated carbon on toxicity abatement. Sci. Total Environ. 2017, 590–591, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Comninellis, C.; Kapalka, A.; Malato, S.; Parsons, S.A.; Poulios, I.; Mantzavinos, D. Advanced oxidation processes for water treatment: Advances and trends for R&D. J. Chem. Technol. Biotechnol. 2008, 83, 769–776. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Kang, J.; Nghiem, L.D.; Price, W.E.; Guo, W.; Ngo, H.H.; Tung, K.L. Comparison between sequential and simultaneous application of activated carbon with membrane bioreactor for trace organic contaminant removal. Bioresour. Technol. 2013, 130, 412–417. [Google Scholar] [CrossRef] [Green Version]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.D.; Haag, W.R. Rate constants for direct reactions of ozone with several drinking water contaminants. Water Res. 1991, 25, 761–773. [Google Scholar] [CrossRef]
- Tizaoui, C.; Grima, N.; Hilal, N. Degradation of the antimicrobial triclocarban (TCC) with ozone. Chem. Eng. Process. Process Intensif. 2011, 50, 637–643. [Google Scholar] [CrossRef]
- D5919-96(2017). Standard Practice for Determination of Adsorptive Capacity of Activated Carbon by a Micro-Isotherm Technique for Adsorbates at ppb Concentrations. Available online: https://pacslabs.com/activated-carbon/astm-method-numbers/ (accessed on 22 May 2021). [CrossRef]
- Dietrich, M.; Andaluri, G.; Smith, R.C.; Suri, R. Combined Ozone and Ultrasound for the Removal of 1,4-Dioxane from Drinking Water. Ozone Sci. Eng. 2017, 39, 244–254. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Bernal-Romero del Hombre Bueno, M.d.l.Á.; Boluda-Botella, N.; Prats Rico, D. Removal of emerging pollutants in water treatment plants: Adsorption of methyl and propylparaben onto powdered activated carbon. Adsorption 2019. [Google Scholar] [CrossRef]
- Flores Baquero, O. Design of an Activated Carbon Columns Plant for Studying Micro-Pollutants Removal. Available online: http://bibing.us.es/proyectos/abreproy/20087/fichero (accessed on 26 February 2021).
- Contreras, J.; Fuenmayorls, H.; Colina, M.; Díazl, A.; Fernández, N.; Pérez De Scow, J.; Colina, G. Capacidad adsortiva del carbón activado preparado a partir del bagazo de la caña de azúcar para la adsorción de fenol, 2-clorofenol, 2-nitrafenol y 2,4-dimetilfenol Adsorptive ca acity of activated carbon derived from cugar cane fagasse for the adsorption. ClENCiA 2008, 16, 111–121. [Google Scholar]
- Aylas Orejón, E.J.; Picasso Escobar, G.; del Rosario Sun Kou, M. Modificación Ácida Del Carbón Activado Y La Influencia Del Grupo Sustituyente En La Adsorción De Compuestos Fenólicos. Rev. La Soc. Química Del Perú 2016, 82, 339–351. [Google Scholar] [CrossRef]
- Nam, S.W.; Choi, D.J.; Kim, S.K.; Her, N.; Zoh, K.D. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J. Hazard. Mater. 2014, 270, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Boehler, M.; Zwickenpflug, B.; Hollender, J.; Ternes, T.; Joss, A.; Siegrist, H. Removal of micropollutants in municipal wastewater treatment plants by powder-activated carbon. Water Sci. Technol. 2012, 66, 2115–2121. [Google Scholar] [CrossRef]
- Kovalova, L.; Siegrist, H.; Von Gunten, U.; Eugster, J.; Hagenbuch, M.; Wittmer, A.; Moser, R.; McArdell, C.S. Elimination of micropollutants during post-treatment of hospital wastewater with powdered activated carbon, ozone, and UV. Environ. Sci. Technol. 2013, 47, 7899–7908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suriyanon, N.; Punyapalakul, P.; Ngamcharussrivichai, C. Mechanistic study of diclofenac and carbamazepine adsorption on functionalized silica-based porous materials. Chem. Eng. J. 2013, 214, 208–218. [Google Scholar] [CrossRef]
- Meinel, F.; Zietzschmann, F.; Ruhl, A.S.; Sperlich, A.; Jekel, M. The benefits of powdered activated carbon recirculation for micropollutant removal in advanced wastewater treatment. Water Res. 2016, 91, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Margot, J.; Kienle, C.; Magnet, A.; Weil, M.; Rossi, L.; de Alencastro, L.F.; Abegglen, C.; Thonney, D.; Chèvre, N.; Schärer, M.; et al. Treatment of micropollutants in municipal wastewater: Ozone or powdered activated carbon? Sci. Total Environ. 2013, 461–462, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J.; Ruhl, A.S.; Zietzschmann, F.; Jekel, M. Direct comparison of ozonation and adsorption onto powdered activated carbon for micropollutant removal in advanced wastewater treatment. Water Res. 2014, 55, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Stoquart, C.; Vázquez Rodríguez, G.A.; Servais, P.; Sauvé, S.; Barbeau, B. Micropollutant Removal Potential by Aged Powdered Activated Carbon. J. Environ. Eng. 2016, 142. [Google Scholar] [CrossRef]
- Apopei, P.; Orha, C.; Popescu, M.I.; Lazau, C.; Manea, F.; Catrinescu, C.; Teodosiu, C. Diclofenac removal from water by photocatalysis- assisted filtration using activated carbon modified with N-doped TiO2. Process Saf. Environ. Prot. 2020, 138, 324–336. [Google Scholar] [CrossRef]
- Ayisha Sidiqua, M.; Priya, V.S. Removal of yellow dye using composite binded adsorbent developed using natural clay and activated carbon from sapindus seed. Biocatal. Agric. Biotechnol. 2021, 33, 101965. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, H. Activated carbon from sawdust for naphthalene removal from contaminated water. Environ. Technol. Innov. 2020, 20, 101080. [Google Scholar] [CrossRef]
- Gandhi, N.; Sirisha, D.; Chandra Sekhar, K.B. Adsorption of Fluoride (F-) from Aqueous Solution by Using Pineapple (Ananas comosus) Peel and Orange (Citrus sinensis) Peel Powders. Int. J. Environ. Bioremediation Biodegrad. 2016, 4, 55–67. [Google Scholar] [CrossRef]
- Gad, H.M.H.; El-Sayed, A.A. Activated carbon from agricultural by-products for the removal of Rhodamine-B from aqueous solution. J. Hazard. Mater. 2009, 168, 1070–1081. [Google Scholar] [CrossRef]
- Blackbeard, J.; Lloyd, J.; Magyar, M.; Mieog, J.; Linden, K.G.; Lester, Y. Demonstrating organic contaminant removal in an ozone-based water reuse process at full scale. Environ. Sci. Water Res. Technol. 2016, 2, 213–222. [Google Scholar] [CrossRef]
- Mathon, B.; Coquery, M.; Miege, C.; Penru, Y.; Choubert, J.M. Removal efficiencies and kinetic rate constants of xenobiotics by ozonation in tertiary treatment. Water Sci. Technol. 2017, 75, 2737–2746. [Google Scholar] [CrossRef]
- Lester, Y.; Mamane, H.; Zucker, I.; Avisar, D. Treating wastewater from a pharmaceutical formulation facility by biological process and ozone. Water Res. 2013, 47, 4349–4356. [Google Scholar] [CrossRef]
- Rosario-Ortiz, F.L.; Wert, E.C.; Snyder, S.A. Evaluation of UV/H2O2 treatment for the oxidation of pharmaceuticals in wastewater. Water Res. 2010, 44, 1440–1448. [Google Scholar] [CrossRef]
- Andreozzi, R.; Marotta, R.; Pinto, G.; Pollio, A. Carbamazepine in water: Persistence in the environment, ozonation treatment and preliminary assessment on algal toxicity. Water Res. 2002, 36, 2869–2877. [Google Scholar] [CrossRef]
- Justo, A.; González, O.; Aceña, J.; Pérez, S.; Barceló, D.; Sans, C.; Esplugas, S. Pharmaceuticals and organic pollution mitigation in reclamation osmosis brines by UV/H2O2 and ozone. J. Hazard. Mater. 2013, 263, 268–274. [Google Scholar] [CrossRef]
- Mcdowell, D.C.; Huber, M.M.; Wagner, M.; Von Gunten, U.; Ternes, T.A. Ozonation of carbamazepine in drinking water: Identification and kinetic study of major oxidation products. Environ. Sci. Technol. 2005, 39, 8014–8022. [Google Scholar] [CrossRef]
- Hübner, U.; Seiwert, B.; Reemtsma, T.; Jekel, M. Ozonation products of carbamazepine and their removal from secondary effluents by soil aquifer treatment—Indications from column experiments. Water Res. 2014, 49, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Buleté, A.; Vulliet, E.; Deshayes, S.; Zedek, S.; Mirande-Bret, C.; Eudes, V.; Bressy, A.; et al. Removal of a wide range of emerging pollutants from wastewater treatment plant discharges by micro-grain activated carbon in fluidized bed as tertiary treatment at large pilot scale. Sci. Total Environ. 2016, 542, 983–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reungoat, J.; Escher, B.I.; Macova, M.; Argaud, F.X.; Gernjak, W.; Keller, J. Ozonation and biological activated carbon filtration of wastewater treatment plant effluents. Water Res. 2012, 46, 863–872. [Google Scholar] [CrossRef]
- Huber, M.M.; Canonica, S.; Park, G.Y.; Von Gunten, U. Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Benner, J.; Salhi, E.; Ternes, T.; von Gunten, U. Ozonation of reverse osmosis concentrate: Kinetics and efficiency of beta blocker oxidation. Water Res. 2008, 42, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kovalova, L.; McArdell, C.S.; von Gunten, U. Prediction of micropollutant elimination during ozonation of a hospital wastewater effluent. Water Res. 2014, 64, 134–148. [Google Scholar] [CrossRef] [Green Version]
- Alharbi, S.K.; Price, W.E.; Kang, J.; Fujioka, T.; Nghiem, L.D. Ozonation of carbamazepine, diclofenac, sulfamethoxazole and trimethoprim and formation of major oxidation products. Desalin. Water Treat. 2016, 57, 29340–29351. [Google Scholar] [CrossRef]
- Yu, H.; Nie, E.; Xu, J.; Yan, S.; Cooper, W.J.; Song, W. Degradation of Diclofenac by Advanced Oxidation and Reduction Processes: Kinetic Studies, Degradation Pathways and Toxicity Assessments. Water Res. 2013, 47, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.D.; Sans, C.; Agüera, A.; Gómez, M.J.; Esplugas, S.; Dezotti, M. Effects of ozone pre-treatment on diclofenac: Intermediates, biodegradability and toxicity assessment. Sci. Total Environ. 2009, 407, 3572–3578. [Google Scholar] [CrossRef] [PubMed]
- Vogna, D.; Marotta, R.; Napolitano, A.; Andreozzi, R.; D’Ischia, M. Advanced oxidation of the pharmaceutical drug diclofenac with UV/H2O2 and ozone. Water Res. 2004, 38, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Estrada, L.A.; Malato, S.; Gernjak, W.; Agüera, A.; Thurman, E.M.; Ferrer, I.; Fernández-Alba, A.R. Photo-fenton degradation of diclofenac: Identification of main intermediates and degradation pathway. Environ. Sci. Technol. 2005, 39, 8300–8306. [Google Scholar] [CrossRef]
- Calza, P.; Sakkas, V.A.; Medana, C.; Baiocchi, C.; Dimou, A.; Pelizzetti, E.; Albanis, T. Photocatalytic degradation study of diclofenac over aqueous TiO2 suspensions. Appl. Catal. B Environ. 2006, 67, 197–205. [Google Scholar] [CrossRef]
- Hofmann, J.; Freier, U.; Wecks, M.; Hohmann, S. Degradation of diclofenac in water by heterogeneous catalytic oxidation with H2O2. Appl. Catal. B Environ. 2007, 70, 447–451. [Google Scholar] [CrossRef]
- Bartels, P.; von Tümpling, W. Solar radiation influence on the decomposition process of diclofenac in surface waters. Sci. Total Environ. 2007, 374, 143–155. [Google Scholar] [CrossRef]
- Xiong, Z.; Cheng, X.; Sun, D. Pretreatment of heterocyclic pesticide wastewater using ultrasonic/ozone combined process. J. Environ. Sci. 2011, 23, 725–730. [Google Scholar] [CrossRef]
- Chen, W.R.; Wu, C.; Elovitz, M.S.; Linden, K.G.; Suffet, I.M. Reactions of thiocarbamate, triazine and urea herbicides, RDX and benzenes on EPA Contaminant Candidate List with ozone and with hydroxyl radicals. Water Res. 2008, 42, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Buffle, M.O.; Von Gunten, U. Phenols and amine induced HO. generation during the initial phase of natural water ozonation. Environ. Sci. Technol. 2006, 40, 3057–3063. [Google Scholar] [CrossRef]
- Zhuan, R.; Wang, J. Degradation of diclofenac in aqueous solution by ionizing radiation in the presence of humic acid. Sep. Purif. Technol. 2020, 234, 116079. [Google Scholar] [CrossRef]
- Li, X.; Hai, F.I.; Nghiem, L.D. Simultaneous activated carbon adsorption within a membrane bioreactor for an enhanced micropollutant removal. Bioresour. Technol. 2011, 102, 5319–5324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Yaohari, H.; De Araujo, C.; Sze, C.C.; Stuckey, D.C. Removal of selected pharmaceuticals in an anaerobic membrane bioreactor (AnMBR) with/without powdered activated carbon (PAC). Chem. Eng. J. 2017, 321, 335–345. [Google Scholar] [CrossRef]
- Viegas, R.M.C.; Mesquita, E.; Campinas, M.; Rosa, M.J. Pilot studies and cost analysis of hybrid powdered activated carbon/ceramic microfiltration for controlling pharmaceutical compounds and organic matter in water reclamation. Water 2020, 12, 33. [Google Scholar] [CrossRef] [Green Version]
- Im, J.K.; Cho, I.H.; Kim, S.K.; Zoh, K.D. Optimization of carbamazepine removal in O3/UV/H2O2 system using a response surface methodology with central composite design. Desalination 2012, 285, 306–314. [Google Scholar] [CrossRef]
- Wang, H.; Zhan, J.; Yao, W.; Wang, B.; Deng, S.; Huang, J.; Yu, G.; Wang, Y. Comparison of pharmaceutical abatement in various water matrices by conventional ozonation, peroxone (O3/H2O2), and an electro-peroxone process. Water Res. 2018, 130, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Von Gunten, U. Oxidation Processes in Water Treatment: Are We on Track? Environ. Sci. Technol. 2018, 52, 5062–5075. [Google Scholar] [CrossRef] [PubMed]




| CEC | Molecule Size (nm) | Molar Mass (g mol−1) | Solubility in Water (mg L−1) | Log Kow | pKa |
|---|---|---|---|---|---|
CBZ | 0.84 | 236.27 | 18.00 (25 °C) | 2.3 | 13.9 |
DCF | 0.93 | 296.10 | 2.37 (25 °C) | 4.51 | 4.15 |
| CEC (mg L−1) | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|
| KF (L g−1) | n | R2 | qe (mg g−1) | b (L mg−1) | R2 | |
| DCF 0.7 | 192.24 | 4.42 | 0.998 | 89.08 | 827.02 | 0.817 |
| DCF 1.8 | 167.13 | 4.06 | 0.990 | 144.32 | 56.09 | 0.811 |
| CBZ 0.7 | 68.70 | 11.17 | 0.990 | 57.41 | 1254.14 | 0.846 |
| CBZ 1.8 | 80.42 | 4.39 | 0.990 | 99.93 | 4.34 | 0.957 |
| CEC (mg L−1) | Pseudo-First-Order Model | Pseudo-Second-Order Model | |||||
|---|---|---|---|---|---|---|---|
| qe (mg g−1) | k1 10−3 (min−1) | R2 | qe (mg g−1) | h (mg g−1 min−1) | k2 10−4 (g mg−1 min−1) | R2 | |
| DCF 0.7 | 20.590 | 23.19 | 0.870 | 52.550 | 5.978 | 21.60 | 1.000 |
| DCF 1.8 | 51.261 | 5.374 | 0.735 | 115.864 | 5.419 | 4.04 | 1.000 |
| CBZ 0.7 | 29.615 | 2.118 | 0.932 | 47.162 | 0.588 | 2.64 | 0.997 |
| CBZ 1.8 | 40.398 | 1.243 | 0.765 | 83.411 | 1.464 | 2.10 | 0.996 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldeguer Esquerdo, A.; Varo Galvañ, P.J.; Sentana Gadea, I.; Prats Rico, D. Carbamazepine and Diclofenac Removal Double Treatment: Oxidation and Adsorption. Int. J. Environ. Res. Public Health 2021, 18, 7163. https://doi.org/10.3390/ijerph18137163
Aldeguer Esquerdo A, Varo Galvañ PJ, Sentana Gadea I, Prats Rico D. Carbamazepine and Diclofenac Removal Double Treatment: Oxidation and Adsorption. International Journal of Environmental Research and Public Health. 2021; 18(13):7163. https://doi.org/10.3390/ijerph18137163
Chicago/Turabian StyleAldeguer Esquerdo, Alejandro, Pedro José Varo Galvañ, Irene Sentana Gadea, and Daniel Prats Rico. 2021. "Carbamazepine and Diclofenac Removal Double Treatment: Oxidation and Adsorption" International Journal of Environmental Research and Public Health 18, no. 13: 7163. https://doi.org/10.3390/ijerph18137163