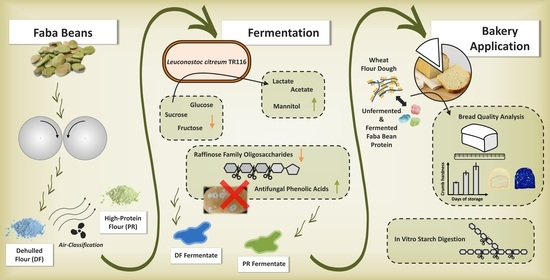
Leuconostoc citreum TR116 as a Microbial Cell Factory to Functionalise High-Protein Faba Bean Ingredients for Bakery Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Sourdough-Like Fermentates
2.3. Characterisation of Fermentation Profile
2.3.1. Determination of Cell Count, pH and Total Titratable Acids
2.3.2. Preparation of Fermentates for Extraction
2.3.3. Levels of Organic Acids and Carbohydrates
2.3.4. Level of Antifungal Compounds
2.4. Application of Sourdough-Like Fermentates and Product Analysis
2.4.1. Flour Analysis
2.4.2. Recipe Adaptation and Bread Production
2.4.3. Determination of Dough pH
2.4.4. Bread Quality Analysis
2.4.5. Scanning Electron Microscopy
2.5. In Vitro Starch Digestion
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fermentation Profile
3.1.1. Microbial Growth and Acidification
3.1.2. Consumption and Degradation of Saccharides and Mannitol
3.1.3. Contents of Antifungal Phenolic Acids
3.2. Baking Properties of Sourdough-Like Fermentates
3.2.1. Water Absorption and Gluten Aggregation
3.2.2. Bread Quality
3.3. In Vitro Starch Digestibility and Indication of Glycaemic Index
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANC | Antinutritional compound |
| ANOVA | Analysis of variance |
| BU | Brabender units |
| CA | Correlation analysis |
| CFU | Cell forming units |
| CWB | Control wheat bread |
| DF | Faba bean dehulled flour |
| DFB | Wheat bread containing DF |
| DFSD | DF fermented with Ln. citreum TR116 starting from inoculation (0 h) |
| DFSDB | Wheat bread containing DFSD |
| DMSO | Dimethylsulfoxide |
| DNS | 3,5-Dinitrosalicylic acid |
| DY | Dough yield |
| EPS | Exopolysaccharides |
| FB | Faba bean |
| FQ | Fermentation quotient |
| FU | Farinograph units |
| FWA | Farinograph water absorption |
| GI | Glycaemic index |
| GL | Glycaemic load |
| HI | Hydrolysis index |
| HPI | High-protein ingredient |
| LAB | Lactic acid bacteria |
| L crumb | Lightness of crumb in the CIE L a b colour space |
| L crust | Lightness of crust in the CIE L a b colour space |
| MRS | deMan-Rogosa-Sharpe |
| PMT | Peak maximum time |
| PR | Air-classified faba bean high-protein flour |
| PRB | Wheat bread containing PR |
| PRSD | PR fermented with Ln. citreum TR116 starting from inoculation (0 h) |
| PRSDB | Wheat bread containing PRSD |
| QuEChERS | quick, easy, cheap, effective, rugged and safe |
| RFO | Raffinose family oligosaccharides |
| SEM | Scanning electron microscopy |
| SPE | Solid phase extraction |
| SV | Specific volume |
| TM | Torque maximum |
| TTA | Total titratable acids |
| %DM | Percentage based on dry matter |
References
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet Commisions 2019, 18, 1–47. [Google Scholar] [CrossRef]
- Frison, E.A. From Uniformity to Diversity: A Paradigm Shift from Industrial Agriculture to Diversified Agroecological Systems; Technical Report 3; International Panel of Experts on Sustainable Food Systems: Louvain-la-Neuve, Belgium, 2016. [Google Scholar] [CrossRef]
- Ranganathan, J.; Vennard, D.; Waite, R.; Searchinger, T.; Dumas, P.; Lipinski, B. Shifting Diets: Toward a Sustainable Food Future; Global Food Policy Report; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2016; Chapter 8; pp. 66–79. [Google Scholar]
- Friedman, M. Nutritional Value of Proteins from Different Food Sources. A Review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- Sosulski, F.W.; Imafidon, G.I. Amino Acid Composition and Nitrogen-to-Protein Conversion Factors for Animal and Plant Foods. J. Agric. Food Chem. 1990, 38, 1351–1356. [Google Scholar] [CrossRef]
- Dewettinck, K.; Van Bockstaele, F.; Kühne, B.; Van de Walle, D.; Courtens, T.M.; Gellynck, X. Nutritional Value of Bread: Influence of Processing, Food Interaction and Consumer Perception. J. Cereal Sci. 2008, 48, 243–257. [Google Scholar] [CrossRef]
- Lopez, H.; Adam, A.; Leenhardt, F.; Scalbert, A.; Remesy, C. Control of The Nutritional Value of Bread. Ind. des Cereal. 2001, 124, 15–20. [Google Scholar]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Augustin, L.S. Too Much Sugar, Too Much Carbohydrate, or just Too Much? Am. J. Clin. Nutr. 2004, 79, 711–712. [Google Scholar] [CrossRef] [PubMed]
- Bugusu, B.A.; Hamaker, B.R.; Rajwa, B. Interaction of Maize Zein with Wheat Gluten in Composite Dough and Bread as Determined by Confocal Laser Scanning Microscopy. Scanning 2002, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Penella, J.M.; Wronkowska, M.; Soral-Smietana, M.; Haros, M. Effect of Whole Amaranth Flour on Bread Properties and Nutritive Value. LWT-Food Sci. Technol. 2013, 50, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Hoehnel, A.; Axel, C.; Bez, J.; Arendt, E.K.; Zannini, E. Comparative Analysis of Plant-Based High-Protein Ingredients and Their Impact on Quality of High-Protein Bread. J. Cereal Sci. 2019, 89, 1–8. [Google Scholar] [CrossRef]
- Hoehnel, A.; Bez, J.; Petersen, I.L.; Amarowicz, R.; Juśkiewicz, J.; Arendt, E.K.; Zannini, E. Enhancing the Nutritional Profile of Regular Wheat Bread While Maintaining Technological Quality and Adequate Sensory Attributes. Food Funct. 2020, 11, 4732–4751. [Google Scholar] [CrossRef]
- Villarino, C.B.; Jayasena, V.; Coorey, R.; Chakrabarti-Bell, S.; Johnson, S.K. The Effects of Australian Sweet Lupin (ASL) Variety on Physical Properties of Flours and Breads. LWT-Food Sci. Technol. 2015, 60, 435–443. [Google Scholar] [CrossRef]
- Turfani, V.; Narducci, V.; Durazzo, A.; Galli, V.; Carcea, M. Technological, Nutritional and Functional Properties of Wheat Bread Enriched with Lentil or Carob Flours. LWT-Food Sci. Technol. 2017, 78, 361–366. [Google Scholar] [CrossRef]
- Marchais, L.P.D.; Foisy, M.; Mercier, S.; Villeneuve, S.; Mondor, M. Bread-Making Potential of Pea Protein Isolate Produced by a Novel Ultrafiltration/Diafiltration Process. Procedia Food Sci. 2011, 1, 1425–1430. [Google Scholar] [CrossRef] [Green Version]
- Boukid, F.; Zannini, E.; Carini, E.; Vittadini, E. Pulses for Bread Fortification: A Necessity or a Choice? Trends Food Sci. Technol. 2019, 88, 416–428. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.B.; Hauggaard-Nielsen, H. Faba Bean in Cropping Systems. Field Crops Res. 2010, 115, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Vogelsang-O’Dwyer, M.; Bez, J.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Detzel, A.; Busch, M.; Krueger, M.; O’Mahony, J.A.; Arendt, E.K.; et al. Comparison of Faba Bean Protein Ingredients Produced Using Dry Fractionation and Isoelectric Precipitation: Techno-Functional, Nutritional and Environmental Performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef] [Green Version]
- Henchion, M.; Hayes, M.; Mullen, A.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Schutyser, M.A.; Pelgrom, P.J.; van der Goot, A.J.; Boom, R.M. Dry Fractionation for Sustainable Production of Functional Legume Protein Concentrates. Trends Food Sci. Technol. 2015, 45, 327–335. [Google Scholar] [CrossRef]
- Gilani, G.S.; Xiao, C.W.; Cockell, K.A. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef]
- Coda, R.; Melama, L.; Rizzello, C.G.; Curiel, J.A.; Sibakov, J.; Holopainen, U.; Pulkkinen, M.; Sozer, N. Effect of Air Classification and Fermentation by Lactobacillus plantarum VTT E-133328 on Faba Bean (Vicia faba L.) Flour Nutritional Properties. Int. J. Food Microbiol. 2015, 193, 34–42. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Coda, R.; Wang, Y.; Verni, M.; Kajala, I.; Katina, K.; Laitila, A. Characterization of Indigenous Pediococcus pentosaceus, Leuconostoc kimchii, Weissella cibaria and Weissella confusa for Faba Bean Bioprocessing. Int. J. Food Microbiol. 2019, 302, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Pulkkinen, M.; Coda, R.; Lampi, A.M.; Varis, J.; Katina, K.; Piironen, V. Possibilities of Reducing Amounts of Vicine and Convicine in Faba Bean Suspensions and Sourdoughs. Eur. Food Res. Technol. 2019, 245, 1507–1518. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sorvali, P.; Laitila, A.; Maina, N.H.; Coda, R.; Katina, K. Dextran Produced In Situ as a Tool to Improve the Quality of Wheat-Faba Bean Composite Bread. Food Hydrocoll. 2018, 84, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wang, Y.; Coda, R.; Säde, E.; Tuomainen, P.; Tenkanen, M.; Katina, K. In Situ Synthesis of Exopolysaccharides by Leuconostoc spp. and Weissella spp. and Their Rheological Impacts in Fava Bean Flour. Int. J. Food Microbiol. 2017, 248, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Coda, R.; Shi, Q.; Tuomainen, P.; Katina, K.; Tenkanen, M. Exopolysaccharides Production during the Fermentation of Soybean and Fava Bean Flours by Leuconostoc mesenteroides DSM 20343. J. Agric. Food Chem. 2017, 65, 2805–2815. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Coda, R.; Holopainen-Mantila, U.; Laitila, A.; Katina, K.; Tenkanen, M. Impact of In Situ Produced Exopolysaccharides on Rheology and Texture of Fava Bean Protein Concentrate. Food Res. Int. 2019, 115, 191–199. [Google Scholar] [CrossRef]
- Shin, S.Y.; Han, N.S. Leuconostoc spp. as Starters and Their Beneficial Roles in Fermented Foods. In Beneficial Microorganisms Food Nutraceuticals; Min-Tze, L., Ed.; Springer: Cham, Switzerland, 2015; pp. 111–132. [Google Scholar] [CrossRef] [Green Version]
- Hemme, D.; Foucaud-Scheunemann, C. Leuconostoc, Characteristics, Use in Dairy Technology and Prospects in Functional Foods. Int. Dairy J. 2004, 14, 467–494. [Google Scholar] [CrossRef]
- Otgonbayar, G.E.; Eom, H.J.; Kim, B.S.; Ko, J.H.; Han, N.S. Mannitol Production by Leuconostoc citreum KACC 91348P Isolated from Kimchi. J. Microbiol. Biotechnol. 2011, 21, 968–971. [Google Scholar] [CrossRef] [Green Version]
- Patra, F.; Tomar, S.K.; Rajput, Y.S.; Singh, R. Characterization of Mannitol Producing Strains of Leuconostoc species. World J. Microbiol. Biotechnol. 2011, 27, 933–939. [Google Scholar] [CrossRef]
- Rice, T.; Sahin, A.W.; Lynch, K.M.; Arendt, E.K.; Coffey, A. Isolation, Characterisation and Exploitation of Lactic Acid Bacteria Capable of Efficient Conversion of Sugars to Mannitol. Int. J. Food Microbiol. 2020, 321, 108546. [Google Scholar] [CrossRef]
- Rice, T.; Sahin, A.W.; Heitmann, M.; Lynch, K.M.; Jacob, F.; Arendt, E.K.; Coffey, A. Application of Mannitol Producing Leuconostoc citreum TR116 to Reduce Sugar Content of Barley, Oat and Wheat Malt-Based Worts. Food Microbiol. 2020, 90, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sahin, A.W.; Rice, T.; Zannini, E.; Axel, C.; Coffey, A.; Lynch, K.M.; Arendt, E.K. Leuconostoc citreum TR116: In-Situ Production of Mannitol in Sourdough and Its Application to Reduce Sugar in Burger Buns. Int. J. Food Microbiol. 2019, 302, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Sahin, A.W.; Rice, T.; Zannini, E.; Lynch, K.M.; Coffey, A.; Arendt, E.K. The Incorporation of Sourdough in Sugar-Reduced Biscuits: A Promising Strategy to Improve Techno-Functional and Sensory Properties. Eur. Food Res. Technol. 2019, 245, 1841–1854. [Google Scholar] [CrossRef]
- Sahin, A.W.; Rice, T.; Zannini, E.; Lynch, K.M.; Coffey, A.; Arendt, E.K. Sourdough Technology as a Novel Approach to Overcome Quality Losses in Sugar-Reduced Cakes. Food Funct. 2019, 10, 4985–4997. [Google Scholar] [CrossRef] [PubMed]
- AACC Method 02-31.01 Titratable Acidity. In AACC Approved Methods of Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1999. [CrossRef]
- AACC Method 44-15.02 Moisture–Air-Oven Methods. In AACC Approved Methods of Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1999. [CrossRef]
- Ispiryan, L.; Heitmann, M.; Hoehnel, A.; Zannini, E.; Arendt, E. Optimization and Validation of an HPAEC-PAD Method for the Quantification of FODMAPs in Cereals and Cereal-Based Products. J. Agric. Food Chem. 2019, 67, 4384–4392. [Google Scholar] [CrossRef]
- Axel, C.; Brosnan, B.; Zannini, E.; Peyer, L.C.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal Activities of Three Different Lactobacillus Species and Their Production of Antifungal Carboxylic Acids in Wheat Sourdough. Appl. Microbiol. Biotechnol. 2016, 100, 1701–1711. [Google Scholar] [CrossRef]
- Broberg, A.; Jacobsson, K.; Ström, K.; Schnürer, J. Metabolite Profiles of Lactic Acid Bacteria in Grass Silage. Appl. Environ. Microbiol. 2007, 73, 5547–5552. [Google Scholar] [CrossRef] [Green Version]
- Aziz, N.H.; Farag, S.E.; Mousa, L.A.; Abo-Zaid, M.A. Comparative Antibacterial and Antifungal Effects of Some Phenolic Compounds. Microbios 1998, 93, 43–54. [Google Scholar]
- Ryan, L.A.; Zannini, E.; Dal Bello, F.; Pawlowska, A.; Koehler, P.; Arendt, E.K. Lactobacillus amylovorus DSM 19280 as a Novel Food-Grade Antifungal Agent for Bakery Products. Int. J. Food Microbiol. 2011, 146, 276–283. [Google Scholar] [CrossRef]
- Brosnan, B.; Coffey, A.; Arendt, E.K.; Furey, A. The QuEChERS Approach in a Novel Application for the Identification of Antifungal Compounds Produced by Lactic Acid Bacteria Cultures. Talanta 2014, 129, 364–373. [Google Scholar] [CrossRef]
- Brennan, C.S.; Tudorica, C.M. Evaluation of Potential Mechanisms by Which Dietary Fibre Additions Reduce the Predicted Glycaemic Index of Fresh Pastas. Int. J. Food Sci. Technol. 2008, 43, 2151–2162. [Google Scholar] [CrossRef]
- Al-Dabbas, M.M.; Al-Ismail, K.; Taleb, R.A.; Ibrahim, S. Acid-Base Buffering Properties of Five Legumes and Selected Food In Vitro. Am. J. Agric. Biol. Sci. 2010, 5, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Chang, R. Physical Chemistry for The Biosciences; University Science Books: Sausalito, CA, USA, 2005. [Google Scholar]
- Han, J.; Hang, F.; Guo, B.; Liu, Z.; You, C.; Wu, Z. Dextran Synthesized by Leuconostoc mesenteroides BD1710 in Tomato Juice Supplemented with Sucrose. Carbohydr. Polym. 2014, 112, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Coffey, A.; Arendt, E.K. Exopolysaccharide Producing Lactic Acid Bacteria: Their Techno-Functional Role and Potential Application in Gluten-Free Bread Products. Food Res. Int. 2018, 110, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Dols, M.; Chraibi, W.; Remaud-Simeon, M.; Lindley, N.D.; Monsan, P.F. Growth and Energetics of Leuconostoc mesenteroides NRRL B-1299 during Metabolism of Various Sugars and Their Consequences for Dextransucrase Production. Appl. Environ. Microbiol. 1997, 63, 2159–2165. [Google Scholar] [CrossRef] [Green Version]
- Vandamme, E.J.; Van Loo, J.; De Laporte, A. Dynamics and Regulation of Sucrose Phosphorylase Formation in Leuconostoc mesenteroides Fermentations. Biotechnol. Bioeng. 1987, 29, 8–15. [Google Scholar] [CrossRef]
- Cogan, T.M.; Jordan, K.N. Metabolism of Leuconostoc Bacteria. J. Dairy Sci. 1994, 77, 2704–2717. [Google Scholar] [CrossRef]
- Erten, H. Metabolism of Fructose as an Electron Acceptor by Leuconostoc mesenteroides. Process Biochem. 1998, 33, 735–739. [Google Scholar] [CrossRef]
- Wisselink, H.W.; Weusthuis, R.A.; Eggink, G.; Hugenholtz, J.; Grobben, G.J. Mannitol Production by Lactic Acid Bacteria: A Review. Int. Dairy J. 2002, 12, 151–161. [Google Scholar] [CrossRef]
- Galle, S.; Schwab, C.; Arendt, E.; Gänzle, M. Exopolysaccharide-Forming Weissella Strains as Starter Cultures for Sorghum and Wheat Sourdoughs. J. Agric. Food Chem. 2010, 58, 5834–5841. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Frias, J.; Vidal-Valverde, C. Alpha-Galactosides: Antinutritional Factors or Functional Ingredients? Crit. Rev. Food Sci. Nutr. 2008, 48, 301–316. [Google Scholar] [CrossRef]
- Muzquiz, M.; Varela, A.; Burbano, C.; Cuadrado, C.; Guillamón, E.; Pedrosa, M.M. Bioactive Compounds in Legumes: Pronutritive and Antinutritive Actions. Implications for Nutrition and Health. Phytochem. Rev. 2012, 11, 227–244. [Google Scholar] [CrossRef]
- Dey, M.; Pridham, J.B.; Holloway, R. Purification and Properties of α-Galactosidases from Vicia faba Seeds. Biochem. J. 1969, 113, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.G.; Follador, R. Metabolism of Oligosaccharides and Starch in Lactobacilli: A Review. Front. Microbiol. 2012, 3, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duskiewicz-Reinhard, W.; Gujska, E.; Khan, K. Reduction of Stachyose in Legume Flours by Lactic Acid Bacteria. J. Food Sci. 1994, 59, 115–117. [Google Scholar] [CrossRef]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Somboonpanyakul, P. B Vitamins and Prebiotic Fructooligosaccharides of Cashew Apple Fermented with Probiotic Strains Lactobacillus spp., Leuconostoc mesenteroides and Bifidobacterium longum. Process Biochem. 2018, 70, 9–19. [Google Scholar] [CrossRef]
- Carr, F.J.; Chill, D.; Maida, N. The Lactic Acid Bacteria: A Literature Survey. Crit. Rev. Microbiol. 2002, 28, 281–370. [Google Scholar] [CrossRef]
- Takahashi, M.; Okada, S.; Uchimura, T.; Kozaki, M. Leuconostoc amelibiosum Schillinger, Holzapfel, and Kandler 1989 Is a Later Subjective Synonym of Leuconostoc citreum Farrow, Facklam, and Collins 1989. Int. J. Syst. Bacteriol. 1992, 42, 649–651. [Google Scholar] [CrossRef] [Green Version]
- Saeed, F.; Pasha, I.; Anjum, F.M.; Sultan, M.T. Arabinoxylans and Arabinogalactans: A Comprehensive Treatise. Crit. Rev. Food Sci. Nutr. 2011, 51, 467–476. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic Acids from Vegetables: A Review on Processing Stability and Health Benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Luo, Y.; Li, J.; Xu, C.; Hao, Z.; Jin, X.; Wang, Q. Impact of Processing on in vitro Bioavailability of Phenols and Flavonodis and Antioxidant Activities in Faba Bean (Vicia faba L.) and Azuki Bean (Vigna angularis L.). Legum. Res. 2014, 37, 492–499. [Google Scholar] [CrossRef]
- Hatcher, D.W.; Kruger, J.E. Simple Phenolic Acids in Flours Prepared from Canadian Wheat: Relationship to Ash Content, Color, and Polyphenol Oxidase Activity. Cereal Chem. 1997, 74, 337–343. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel Insights on The Functional/Nutritional Features of the Sourdough Fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.M.; Brosnan, B.; Furey, A.; Coffey, A.; Zannini, E.; Arendt, E.K. Lactic Acid Bacteria Bioprotection Applied to the Malting Process. Part I: Strain Characterization and Identification of Antifungal Compounds. Food Control 2015, 51, 433–443. [Google Scholar] [CrossRef]
- Ndagano, D.; Lamoureux, T.; Dortu, C.; Vandermoten, S.; Thonart, P. Antifungal Activity of 2 Lactic Acid Bacteria of the Weissella Genus Isolated from Food. J. Food Sci. 2011, 76, M305–M311. [Google Scholar] [CrossRef]
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic Acid Antioxidants: An Electrochemical Overview. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A Review on Antioxidants, Prooxidants and Related Controversy: Natural and Synthetic Compounds, Screening and Analysis Methodologies and Future Perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E.; Rémésy, C. Is the In Vitro Antioxidant Potential of Whole-Grain Cereals and Cereal Products Well Reflected In Vivo? J. Cereal Sci. 2008, 48, 258–276. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Polo, A.; Rizzello, C.G. The Sourdough Fermentation Is the Powerful Process to Exploit the Potential of Legumes, Pseudo-Cereals and Milling By-Products in Baking Industry. Crit. Rev. Food Sci. Nutr. 2019, 60, 1–16. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the Sourdough May Affect the Functional Features of Leavened Baked Goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Gerez, C.L.; Torino, M.I.; Rollán, G.; Font de Valdez, G. Prevention of Bread Mould Spoilage by Using Lactic Acid Bacteria with Antifungal Properties. Food Control 2009, 20, 144–148. [Google Scholar] [CrossRef]
- Axel, C.; Zannini, E.; Arendt, E.K. Mold Spoilage of Bread and Its Biopreservation: A Review of Current Strategies for Bread Shelf Life Extension. Crit. Rev. Food Sci. Nutr. 2017, 57, 3528–3542. [Google Scholar] [CrossRef] [PubMed]
- Peyer, L.C.; Axel, C.; Lynch, K.M.; Zannini, E.; Jacob, F.; Arendt, E.K. Inhibition of Fusarium culmorum by Carboxylic Acids Released from Lactic Acid Bacteria in a Barley Malt Substrate. Food Control 2016, 69, 227–236. [Google Scholar] [CrossRef]
- Ventour, L. Food Waste Report V2: The Food We Waste; Waste & Resources Action Programme: Banbury, UK, 2008; Volume 2, pp. 1–237. [Google Scholar]
- Axel, C.; Röcker, B.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Application of Lactobacillus amylovorus DSM19280 in Gluten-Free Sourdough Bread to Improve the Microbial Shelf Life. Food Microbiol. 2015, 47, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Axel, C.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal Sourdough Lactic Acid Bacteria as Biopreservation Tool in Quinoa and Rice Bread. Int. J. Food Microbiol. 2016, 239, 86–94. [Google Scholar] [CrossRef]
- Cortés-Zavaleta, O.; López-Malo, A.; Hernández-Mendoza, A.; García, H.S. Antifungal Activity of Lactobacilli and Its Relationship with 3-Phenyllactic Acid Production. Int. J. Food Microbiol. 2014, 173, 30–35. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Visconti, A. Antifungal Activity of Phenyllactic Acid against Molds Isolated from Bakery Products. Appl. Environ. Microbiol. 2003, 69, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, N.; Gánzle, M.G.; Vogel, R.F. Influence of Peptide Supply and Cosubstrates on Phenylalanine Metabolism of Lactobacillus sanfranciscensis DSM20451T and Lactobacillus plantarum TMW1.468. J. Agric. Food Chem. 2006, 54, 3832–3839. [Google Scholar] [CrossRef]
- Gänzle, M.G. Enzymatic and Bacterial Conversions during Sourdough Fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and Functional Paths of Lactic Acid Bacteria in Plant Foods: Get Out of the Labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef]
- Omedi, J.O.; Huang, W.; Zheng, J. Effect of Sourdough Lactic Acid Bacteria Fermentation on Phenolic Acid Release and Antifungal Activity in Pitaya Fruit Substrate. LWT-Food Sci. Technol. 2019, 111, 309–317. [Google Scholar] [CrossRef]
- Magalhães, S.C.; Taveira, M.; Cabrita, A.R.; Fonseca, A.J.; Valentão, P.; Andrade, P.B. European Marketable Grain Legume Seeds: Further Insight Into Phenolic Compounds Profiles. Food Chem. 2017, 215, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Baginsky, C.; Peña-Neira, Á.; Cáceres, A.; Hernández, T.; Estrella, I.; Morales, H.; Pertuzé, R. Phenolic Compound Composition in Immature Seeds of Fava Bean (Vicia faba L.) Varieties Cultivated in Chile. J. Food Compos. Anal. 2013, 31, 1–6. [Google Scholar] [CrossRef]
- Bekkara, F.; Jay, M.; Viricel, M.R.; Rome, S. Distribution of Phenolic Compounds within Seed and Seedlings of two Vicia faba cvs Differing in Their Seed Tannin Content, and Study of Their Seed and Root Phenolic Exudations. Plant Soil 1998, 203, 27–36. [Google Scholar] [CrossRef]
- Siah, S.; Konczak, I.; Wood, J.A.; Agboola, S.; Blanchard, C.L. Effects of Roasting on Phenolic Composition and In Vitro Antioxidant Capacity of Australian Grown Faba Beans (Vicia faba L.). Plant Foods Hum. Nutr. 2014, 69, 85–91. [Google Scholar] [CrossRef]
- Sosulski, F.W.; Dabrowski, K.J. Composition of Free and Hydrolyzable Phenolic Acids in the Flours and Hulls of Ten Legume Species. J. Agric. Food Chem. 1984, 32, 131–133. [Google Scholar] [CrossRef]
- Valente, I.M.; Maia, M.R.; Malushi, N.; Oliveira, H.M.; Papa, L.; Rodrigues, J.A.; Fonseca, A.J.; Cabrita, A.R. Profiling of Phenolic Compounds and Antioxidant Properties of European Varieties and Cultivars of Vicia faba L. pods. Phytochemistry 2018, 152, 223–229. [Google Scholar] [CrossRef]
- Herrmann, K. Occurrence and Content of Hydroxycinnamic and Hydroxybenzoic Acid Compounds in Foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 315–347. [Google Scholar] [CrossRef]
- Esteban-Torres, M.; Reverón, I.; Mancheño, J.M.; De las Rivas, B.; Muñoz, R. Characterization of a Feruloyl Esterase from Lactobacillus plantarum. Appl. Environ. Microbiol. 2013, 79, 5130–5136. [Google Scholar] [CrossRef] [Green Version]
- Coda, R.; Kianjam, M.; Pontonio, E.; Verni, M.; Di Cagno, R.; Katina, K.; Rizzello, C.G.; Gobbetti, M. Sourdough-Type Propagation of Faba Bean Flour: Dynamics of Microbial Consortia and Biochemical Implications. Int. J. Food Microbiol. 2017, 248, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Filannino, P.; Gobbetti, M.; De Angelis, M.; Di Cagno, R. Hydroxycinnamic Acids Used as External Acceptors of Electrons: An Energetic Advantage for Strictly Heterofermentative Lactic Acid Bacteria. Appl. Environ. Microbiol. 2014, 80, 7574–7582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Parliament & Council. Regulation (EC) No 1924/2006 of the European Parliament and of the Council on Nutrition and Health Claims Made on Foods; European Parliament & Council: Brussels, Belgium, 2006. [Google Scholar]
- Van Bockstaele, F.; De Leyn, I.; Eeckhout, M.; Dewettinck, K. Rheological Properties of Wheat Flour Dough and the Relationship with Bread Volume. I. Creep-Recovery Measurements. Cereal Chem. 2008, 85, 753–761. [Google Scholar] [CrossRef]
- Wehrle, K.; Grau, H.; Arendt, E.K. Effects of Lactic Acid, Acetic Acid, and Table Salt on Fundamental Rheological Properties of Wheat Dough. Cereal Chem. 1997, 74, 739–744. [Google Scholar] [CrossRef]
- Maher Galal, A.; Varriano-Marston, E.; Johnson, J.A. Rheological Dough Properties as Affected by Organic Acids and Salt. Cereal Chem. 1978, 55, 683–691. [Google Scholar]
- Wehrle, K.; Arendt, E.K. Rheological Changes in Wheat Sourdough during Controlled and Spontaneous Fermentation. Cereal Chem. 1998, 75, 882–886. [Google Scholar] [CrossRef]
- Amoriello, T.; Turfani, V.; Galli, V.; Mellara, F.; Carcea, M. Evaluation of a New Viscometer Performance in Predicting the Technological Quality of Soft Wheat Flour. Cereal Chem. 2016, 93, 364–368. [Google Scholar] [CrossRef]
- Chandi, G.K.; Seetharaman, K. Optimization of Gluten Peak Tester: A Statistical Approach. J. Food Qual. 2012, 35, 69–75. [Google Scholar] [CrossRef]
- Marti, A.; Ulrici, A.; Foca, G.; Quaglia, L.; Pagani, M.A. Characterization of Common Wheat Flours (Triticum aestivum L.) through Multivariate Analysis of Conventional Rheological Parameters and Gluten Peak Test Indices. LWT-Food Sci. Technol. 2015, 64, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Bouachra, S.; Begemann, J.; Aarab, L.; Hüsken, A. Prediction of Bread Wheat Baking Quality Using an Optimized GlutoPeak®-Test Method. J. Cereal Sci. 2017, 76, 8–16. [Google Scholar] [CrossRef]
- Silow, C.; Zannini, E.; Axel, C.; Lynch, K.M.; Arendt, E.K. Effect of Salt Reduction on Wheat-Dough Properties and Quality Characteristics of Puff Pastry with Full and Reduced Fat Content. Food Res. Int. 2016, 89, 330–337. [Google Scholar] [CrossRef]
- Goldstein, A.; Ashrafi, L.; Seetharaman, K. Effects of Cellulosic Fibre on Physical and Rheological Properties of Starch, Gluten and Wheat Flour. Int. J. Food Sci. Technol. 2010, 45, 1641–1646. [Google Scholar] [CrossRef]
- Decock, P.; Cappelle, S. Bread Technology and Sourdough Technology. Trends Food Sci. Technol. 2005, 16, 113–120. [Google Scholar] [CrossRef]
- Purlis, E. Browning Development in Bakery Products—A Review. J. Food Eng. 2010, 99, 239–249. [Google Scholar] [CrossRef]
- Xu, Y.; Pitkänen, L.; Maina, N.H.; Coda, R.; Katina, K.; Tenkanen, M. Interactions between Fava Bean Protein and Dextrans Produced by Leuconostoc pseudomesenteroides DSM 20193 and Weissella cibaria Sj 1b. Carbohydr. Polym. 2018, 190, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Kaditzky, S.; Seitter, M.; Hertel, C.; Vogel, R.F. Performance of Lactobacillus sanfranciscensis TMW 1.392 and Its Levansucrase Deletion Mutant in Wheat Dough and Comparison of Their Impact on Bread Quality. Eur. Food Res. Technol. 2008, 227, 433–442. [Google Scholar] [CrossRef]
- Coda, R.; Varis, J.; Verni, M.; Rizzello, C.G.; Katina, K. Improvement of the Protein Quality of Wheat Bread through Faba Bean Sourdough Addition. LWT-Food Sci. Technol. 2017, 82, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Salméron, J.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Wing, A.L.; Willett, W.C. Dietary Fiber, Glycemic Load, and Risk of Non-insulin-Dependent Diabetes Mellitus in Women. J. Am. Med. Assoc. 1997, 277, 472–477. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Pereira, M.A.; Kroenke, C.H.; Hilner, J.E.; Van Horn, L.; Slattery, M.L.; Jacobs, D.R. Dietary Fiber, Weight Gain, and Cardiovascular Disease Risk Factors in Young Adults. J. Am. Med. Assoc. 1999, 282, 1539–1546. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Willett, W.C.; Stampfer, M.J.; Hu, F.B.; Franz, M.; Sampson, L.; Hennekens, C.H.; Manson, J.A.E. A Prospective Study of Dietary Glycemic Load, Carbohydrate Intake, and Risk of Coronary Hart Disease in US Women. Am. J. Clin. Nutr. 2000, 71, 1455–1461. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, S.; Dal Maso, L.; Augustin, L.; Negri, E.; Parpinel, M.; Boyle, P.; Jenkins, D.; La Vecchia, C. Dietary Glycemic Load and Colorectal Cancer Risk. Ann. Oncol. 2001, 12, 173–178. [Google Scholar] [CrossRef]
- Augustin, L.; Dal Maso, L.; La Vecchia, C.; Parpinel, M.; Negri, E.; Vaccarella, S.; Kendall, C.; Jenkins, D.; Franceschi, S. Dietary Glycemic index and Glycemix Load, and Breast Cancer Risk: A Case-Control Study. Ann. Oncol. 2001, 12, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hamaker, B.R. Slowly Digestible Starch: Concept, Mechanism, and Proposed Extended Glycemic Index. Crit. Rev. Food Sci. Nutr. 2009, 49, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ao, Z.; Hamaker, B.R. Controlling the Delivery of Glucose in Foods. In Designing Functional Foods: Measuring and Controlling Food Structure Breakdown and Nutrient Absorption; Woodhead Publishing: Cambridge, UK, 2009; Chapter 21; pp. 547–571. [Google Scholar] [CrossRef]
- Singh, J.; Dartois, A.; Kaur, L. Starch Digestibility in Food Matrix: A Review. Trends Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Petitot, M.; Abecassis, J.; Micard, V. Structuring of Pasta Components during Processing: Impact on Starch and Protein Digestibility and Allergenicity. Trends Food Sci. Technol. 2009, 20, 521–532. [Google Scholar] [CrossRef]
- Granfeldt, Y.; Björck, I. Glycemic Response to Starch in Pasta: A Study of Mechanisms of Limited Enzyme Availability. J. Cereal Sci. 1991, 14, 47–61. [Google Scholar] [CrossRef]
- Holt, S.H.; Brand-Miller, J.C.; Stitt, P.A. The Effects of Equal-Energy Portions of Different Breads on Blood Glucose Levels, Feelings of Fullness and Subsequent Food Intake. J. Am. Diet. Assoc. 2001, 101, 767–773. [Google Scholar] [CrossRef]
- Liljeberg, H.G.; Lonner, C.H.; Bjorck, I.M. Sourdough Fermentation or Addition of Organic Acids or Corresponding Salts to Bread Improves Nutritional Properties of Starch in Healthy Humans. J. Nutr. 1995, 125, 1503–1511. [Google Scholar] [CrossRef]
- Liljeberg, H.; Björck, I. Delayed Gastric Emptying Rate May Explain Improved Glycaemia in Healthy Subjects to a Starchy Meal with Added Vinegar. Eur. J. Clin. Nutr. 1998, 52, 368–371. [Google Scholar] [CrossRef] [Green Version]
- Scazzina, F.; Del Rio, D.; Pellegrini, N.; Brighenti, F. Sourdough Bread: Starch Digestibility and Postprandial Glycemic Response. J. Cereal Sci. 2009, 49, 419–421. [Google Scholar] [CrossRef]
- Martínez, M.M.; Román, L.; Gómez, M. Implications of Hydration Depletion in the In Vitro Starch Digestibility of White Bread Crumb and Crust. Food Chem. 2018, 239, 295–303. [Google Scholar] [CrossRef]
- Dhital, S.; Warren, F.J.; Butterworth, P.J.; Ellis, P.R.; Gidley, M.J. Mechanisms of Starch Digestion by α-Amylase–Structural Basis for Kinetic Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 875–892. [Google Scholar] [CrossRef] [PubMed]





| Ingredient | % Based on Flour | % Based on Recipe |
|---|---|---|
| Wheat flour | 100.0 (85.0 ) | 60.46 |
| Faba bean ingredient (DF/PR/DFSD/PRSD) | 0.0 (15.00 ) | 0.00 |
| Baker’s yeast | 2.0 | 1.21 |
| NaCl | 2.0 | 1.21 |
| Oil | 1.0 | 0.60 |
| Water | 60.4 (FWA *) | 36.52 |
| Total | 165.4 | 100.00 |
| Ferm. Time [h] | Acetic Acid | Lactic Acid | Fermentation Quotient | |
|---|---|---|---|---|
| Faba bean | 0 | n.d. | n.d. | - |
| dehulled | 6 | 0.45 ± 0.01 | 0.51 ± 0.01 | 0.76 ± 0.03 |
| flour | 12 | 0.69 ± 0.00 | 0.95 ± 0.02 | 0.91 ± 0.02 |
| (DFSD) | 18 | 0.75 ± 0.01 | 1.13 ± 0.02 | 1.00 ± 0.02 |
| 24 | 0.84 ± 0.01 | 1.23 ± 0.02 | 0.98 ± 0.02 | |
| 30 | 0.91 ± 0.01 | 1.25 ± 0.01 | 0.92 ± 0.02 | |
| 36 | 1.01 ± 0.02 | 1.33 ± 0.02 | 0.88 ± 0.03 | |
| 42 | 1.04 ± 0.03 | 1.37 ± 0.04 | 0.88 ± 0.06 | |
| 48 | 1.16 ± 0.10 | 1.32 ± 0.13 | 0.77 ± 0.15 | |
| Faba bean | 0 | n.d. | n.d. | - |
| high-protein | 6 | 0.56 ± 0.01 | 0.64 ± 0.02 | 0.75 ± 0.02 |
| flour | 12 | 1.02 ± 0.00 | 1.13 ± 0.02 | 0.74 ± 0.02 |
| (PRSD) | 18 | 1.07 ± 0.02 | 1.25 ± 0.02 | 0.78 ± 0.02 |
| 24 | 1.14 ± 0.03 | 1.33 ± 0.03 | 0.78 ± 0.04 | |
| 30 | 1.12 ± 0.05 | 1.35 ± 0.04 | 0.80 ± 0.06 | |
| 36 | 1.24 ± 0.06 | 1.41 ± 0.06 | 0.76 ± 0.07 | |
| 42 | 1.29 ± 0.05 | 1.45 ± 0.06 | 0.76 ± 0.07 | |
| 48 | 1.37 ± 0.14 | 1.41 ± 0.13 | 0.70 ± 0.14 |
| Ferm. Time [h] | Sucrose | Glucose | Fructose | Mannitol | Verbascose | Stachyose/Raffinose | Galactose | |
|---|---|---|---|---|---|---|---|---|
| Faba bean | 0 | 1.93 ± 0.05 | n.d. | n.d. | n.d. | 0.90 ± 0.02 | 0.96 ± 0.02 | 0.04 ± 0.00 |
| dehulled | 6 | 0.03 ± 0.00 | 0.07 ± 0.01 | 0.39 ± 0.00 | 0.60 ± 0.01 | 0.84 ± 0.02 | 0.99 ± 0.02 | 0.28 ± 0.01 |
| flour | 12 | n.d. | n.d. | 0.03 ± 0.00 | 0.82 ± 0.02 | 0.72 ± 0.03 | 0.99 ± 0.03 | 0.48 ± 0.01 |
| (DFSD) | 18 | n.d. | n.d. | n.d. | 0.82 ± 0.01 | 0.56 ± 0.02 e | 0.90 ± 0.03 | 0.66 ± 0.02 |
| 24 | n.d. | n.d. | n.d. | 0.77 ± 0.01 | 0.49 ± 0.01 | 0.84 ± 0.01 | 0.75 ± 0.01 | |
| 30 | n.d. | n.d. | n.d. | 0.74 ± 0.01 | 0.42 ± 0.03 | 0.77 ± 0.04 | 0.86 ± 0.02 | |
| 36 | n.d. | n.d. | n.d. | 0.72 ± 0.03 | 0.37 ± 0.01 | 0.71 ± .02 | 0.95 ± 0.01 e | |
| 42 | n.d. | n.d. | n.d. | 0.70 ± 0.02 | 0.32 ± 0.02 | 0.63 ± 0.03 | 1.03 ± 0.03 | |
| 48 | n.d. | n.d. | n.d. | 0.65 ± 0.11 | 0.28 ± 0.01 | 0.59 ± 0.03 | 1.12 ± 0.02 | |
| Faba bean | 0 | 1.42 ± 0.04 | 0.03 ± 0.00 | n.d. | n.d. | 0.84 ± 0.04 | 0.95 ± 0.05 | 0.13 ± >0.01 |
| high-protein | 6 | n.d. | 0.02 ± 0.01 | 0.25 ± 0.03 | 0.51 ± 0.03 | 0.81 ± 0.03 | 1.03 ± 0.04 | 0.46 ± 0.00 |
| flour | 12 | n.d. | n.d. | 0.17 ± 0.02 | 0.58 ± 0.02 | 0.76 ± 0.02 | 1.09 ± 0.03 | 0.69 ± 0.01 |
| (PRSD) | 18 | n.d. | n.d. | n.d. | 0.67 ± 0.02 | 0.63 ± 0.02 | 1.01 ± 0.02 | 0.87 ± 0.03 |
| 24 | n.d. | n.d. | n.d. | 0.63 ± 0.02 | 0.54 ± 0.01 e | 0.93 ± 0.03 | 0.99 ± 0.03 | |
| 30 | n.d. | n.d. | n.d. | 0.63 ± 0.04 | 0.47 ± 0.03 | 0.87 ± 0.04 | 1.10 ± 0.06 | |
| 36 | n.d. | n.d. | n.d. | 0.61 ± 0.04 | 0.42 ± 0.01 | 0.83 ± 0.01 | 1.24 ± 0.02 | |
| 42 | n.d. | n.d. | n.d. | 0.60 ± 0.04 | 0.36 ± 0.01 | 0.75 ± 0.01 | 1.34 ± 0.03 | |
| 48 | n.d. | n.d. | n.d. | 0.52 ± 0.09 | 0.33 ± 0.02 | 0.73 ± 0.04 | 1.38 ± 0.05 |
| Ferm. Time [h] | 4-Hydroxybenzoic Acid | Caffeic Acid | Coumaric Acid | Ferulic Acid | Phenyllactic Acid | |
|---|---|---|---|---|---|---|
| Faba bean | 0 | 3.57 ± 0.05 | n.d. | n.d. | 3.03 ± 0.16 | n.d. |
| dehulled | 6 | 3.80 ± 0.10 | n.d. | n.d. | 3.27 ± 0.21 | n.d. |
| flour | 12 | 3.60 ± 0.16 | 4.80 ± 0.28 | n.d. | 3.25 ± 0.18 | 7.57 ± 0.46 |
| (DFSD) | 18 | 3.87 ± 0.04 | 5.82 ± 0.21 | n.d. | 3.69 ± 0.19 | 10.45 ± 0.21 |
| 24 | 3.93 ± 0.07 | 5.90 ± 0.13 | n.d. | 4.02 ± 0.17 | 13.15 ± 0.14 | |
| 30 | 4.10 ± 0.08 | 5.99 ± 0.07 | 2.52 ± 0.06 e | 4.24 ± 0.153 | 18.32 ± 1.20 | |
| 36 | 4.40 ± 0.10 | 6.41 ± 0.24 | 2.78 ± 0.07 | 4.64 ± 0.15 | 21.27 ± 2.16 | |
| 42 | 4.54 ± 0.11 | 6.69 ± 0.20 | 2.94 ± 0.03 | 5.05 ± 0.26 | 24.02 ± 2.61 | |
| 48 | 4.60 ± 0.08 | 6.45 ± 0.27 | 2.99 ± 0.07 | 5.22 ± 0.18 | 29.40 ± 5.30 | |
| Faba bean | 0 | 4.22 ± 0.53 | n.d. | n.d. | 3.15 ± 0.24 | n.d. |
| high-protein | 6 | 4.15 ± 0.12 | 3.60 ± 0.85 | n.d. | 3.86 ± 0.21 | 3.38 ± 0.08 |
| flour | 12 | 3.20 ± 0.43 | 5.46 ± 0.60 | n.d. | 2.95 ± 0.29 | 7.94 ± 0.98 |
| (PRSD) | 18 | 4.23 ± 0.13 | 7.77 ± 0.14 | 3.95 ± 0.21 | 3.69 ± 0.08 | 14.66 ± 0.66 |
| 24 | 3.75 ± 0.31 | 7.42 ± 0.63 | 4.58 ± 0.43 | 3.65 ± 0.25 | 17.94 ± 2.42 | |
| 30 | 4.27 ± 0.17 | 8.96 ± 0.64 | 5.26 ± 0.71 | 4.11 ± 0.08 | 20.30 ± 1.84 | |
| 36 | 3.83 ± 0.50 | 8.36 ± 1.02 | 5.26 ± 0.99 | 3.98 ± 0.47 | 21.26 ± 3.21 | |
| 42 | 4.43 ± 0.43 | 9.15 ± 0.88 | 5.89 ± 0.94 | 4.97 ± 0.38 | 28.25 ± 3.22 | |
| 48 | 4.23 ± 0.27 | 9.06 ± 0.45 | 6.27 ± 1.03 | 5.14 ± 0.48 | 33.54 ± 7.12 |
| Variable | Wheat Flour | DF Flour Mix | DFSD Flour Mix | PR Flour Mix | PRSD Flour Mix |
|---|---|---|---|---|---|
| Farinograph | |||||
| Farinograph water absorption | |||||
| (FWA) [%] | 60.57 ± 0.09 | 60.73 ± 0.09 | 61.27 ± 0.05 | 59.87 ± 0.12 | 62.43 ± 0.17 |
| GlutoPeak | |||||
| Peak maximum time | |||||
| (PMT) [s] | 45.7 ± 1.1 | 58.5 ± 1.2 | 36.7 ± 1.8 | 29.7 ± 0.8 | 32.0 ± 0.7 |
| Torque maximum | |||||
| (TM) [BU] | 77.0 ± 0.0 | 49.7 ± 1.1 | 56.7 ± 0.4 | 54.7 ± 0.4 | 58.3 ± 1.1 |
| Variable | CWB | DFB | DFSDB | PRB | PRSDB |
|---|---|---|---|---|---|
| pH of dough | 5.76 ± 0.04 | 5.79 ± 0.07 | 5.47 ± 0.02 | 5.77 ± 0.02 | 5.69 ± 0.06 |
| Bake loss [%] | 12.0 ± 0.7 | 11.4 ± 0.5 | 12.8 ± 0.9 | 10.6 ± 0.9 | 12.2 ± 0.9 |
| Specific volume (SV) [ml/g] | 3.09 ± 0.12 | 2.42 ± 0.09 | 2.96 ± 0.10 | 2.16 ± 0.07 e | 2.58 ± 0.09 |
| Hardness day 0 [N] | 6.31 ± 1.01 e | 15.65 ± 1.51 | 8.02 ± 1.01 | 18.50 ± 1.4 | 11.44 ± 1.78 |
| Hardness day 2 [N] | 13.04 ± 1.46 | 30.41 ± 4.13 | 14.79 ± 1.48 | 33.51 ± 2.83 | 19.17 ± 2.26 |
| Hardness day 5 [N] | 20.65 ± 2.57 e | 39.33 ± 3.26 | 22.66 ± 2.59 | 44.53 ± 4.99 | 28.04 ± 2.84 |
| Staling rate [N/d] | 2.31 ± 0.36 | 1.53 ± 0.25 | 1.86 ± 0.43 | 1.42 ± 0.31 | 1.49 ± 0.34 |
| Number of cells | 1527 ± 95 | 1338 ± 94 | 1243 ± 80 | 1454 ± 123 | 1144 ± 129 |
| Cell area [%] | 48.4 ± 1.1 | 46.9 ± 1.0 | 49.6 ± 1.0 | 46.3 ± 1.6 | 49.4 ± 1.0 |
| Lightness of crumb (L crumb) | 76.9 ± 2.7 | 74.7 ± 2.0 | 71.5 ± 1.4 | 73.2 ± 1.9 | 70.6 ± 2.3 |
| Lightness of crust (L crust) | 77.3 ± 2.6 | 70.5 ± 3.8 | 64.7 ± 2.5 | 63.3 ± 5.0 | 61.7 ± 2.5 |
| Variable | CWB | DFB | DFSDB | PRB | PRSDB |
|---|---|---|---|---|---|
| Resistant starch [%DM] | 1.18 ± 0.02 | 1.45 ± 0.16 | 1.26 ± 0.00 | 0.93 ± 0.04 | 0.95 ± 0.04 |
| Digestible starch [%DM] | 70.54 ± 0.65 | 66.75 ± 0.08 | 67.06 ± 0.35 | 61.96 ± 0.12 | 61.96 ± 1.17 |
| Total starch [%DM] | 71.72 ± 0.67 | 68.20 ± 0.08 | 68.32 ± 0.35 | 62.89 ± 0.15 | 62.91 ± 1.13 |
| Hydrolysis index [%] | 100 | 99.9 ± 3.8 | 119.0 ± 3.7 | 133.4 ± 4.4 | 114.9 ± 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoehnel, A.; Bez, J.; Sahin, A.W.; Coffey, A.; Arendt, E.K.; Zannini, E. Leuconostoc citreum TR116 as a Microbial Cell Factory to Functionalise High-Protein Faba Bean Ingredients for Bakery Applications. Foods 2020, 9, 1706. https://doi.org/10.3390/foods9111706
Hoehnel A, Bez J, Sahin AW, Coffey A, Arendt EK, Zannini E. Leuconostoc citreum TR116 as a Microbial Cell Factory to Functionalise High-Protein Faba Bean Ingredients for Bakery Applications. Foods. 2020; 9(11):1706. https://doi.org/10.3390/foods9111706
Chicago/Turabian StyleHoehnel, Andrea, Jürgen Bez, Aylin W. Sahin, Aidan Coffey, Elke K. Arendt, and Emanuele Zannini. 2020. "Leuconostoc citreum TR116 as a Microbial Cell Factory to Functionalise High-Protein Faba Bean Ingredients for Bakery Applications" Foods 9, no. 11: 1706. https://doi.org/10.3390/foods9111706