Seasonal Variation of Glucosinolate Hydrolysis Products in Commercial White and Red Cabbages (Brassica oleracea var. capitata)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Sample Preparation
2.4. Analysis of Glucosinolates
2.5. Determination of Glucosinolate Breakdown Products
2.6. Statistical Analysis
3. Results
3.1. Glucosinolates in White and Red Cabbage from Local Food Retailers
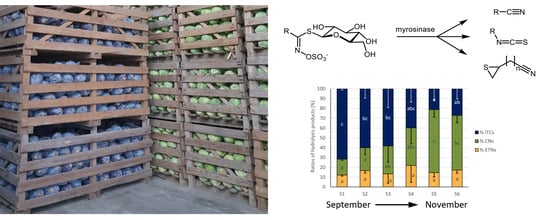
3.2. Glucosinolate Hydrolysis Product Formation in White and Red Cabbages from Local Food Retailers
3.3. Glucosinolates and Glucosinolate Hydrolysis Products Formation in Freshly Harvested White and Red Cabbages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- BMEL-Bundesministerium für Ernährung und Landwirtschaft. Available online: https://www.bmel-statistik.de/fileadmin/daten/GBT-0070004-2018.pdf (accessed on 15 July 2020).
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, D.; Omirou, M.; Liadaki, K.; Tsikou, D.; Delis, C.; Garagounis, C.; Krokida, A.; Zambounis, A.; Papadopoulou, K.K. Glucosinolate biosynthesis in Eruca sativa. Plant Physiol. Biochem. 2016, 109, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and stability of glucosinolates and their breakdown products in foods. Angew. Chem. Int. Ed. 2014, 53, 11430–11450. [Google Scholar] [CrossRef]
- Bell, L.; Oloyede, O.O.; Lignou, S.; Wagstaff, C.; Methven, L. Taste and flavor perceptions of glucosinolates, isothiocyanates, and related compounds. Mol. Nutr. Food Res. 2018, 62, e1700990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koroleva, O.A.; Cramer, R. Single-cell proteomic analysis of glucosinolate-rich S-cells in Arabidopsis thaliana. Methods 2011, 54, 413–423. [Google Scholar] [CrossRef]
- Wittstock, U.; Burow, M. Glucosinolate breakdown in Arabidopsis: Mechanism, regulation and biological significance. Arab. Book 2010, 8, e0134. [Google Scholar] [CrossRef] [Green Version]
- Palliyaguru, D.L.; Yuan, J.-M.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the power of plants to people. Mol. Nutr. Food Res. 2018, 62, e1700965. [Google Scholar] [CrossRef]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An overview of their antimicrobial activity against human infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef] [Green Version]
- Chou, Y.-C.; Chang, M.-Y.; Lee, H.-T.; Shen, C.-C.; Harnod, T.; Liang, Y.-J.; Wu, R.S.-C.; Lai, K.; Hsu, F.; Chung, J.-G. Phenethyl isothiocyanate inhibits in vivo growth of xenograft tumors of human glioblastoma cells. Molecules 2018, 23, 2305. [Google Scholar] [CrossRef] [Green Version]
- Veeranki, O.L.; Bhattacharya, A.; Tang, L.; Marshall, J.R.; Zhang, Y. Cruciferous vegetables, isothiocyanates, and prevention of bladder cancer. Curr. Pharmacol. Rep. 2015, 1, 272–282. [Google Scholar] [CrossRef] [Green Version]
- Hanschen, F.S.; Herz, C.; Schlotz, N.; Kupke, F.; Rodríguez, M.M.B.; Schreiner, M.; Rohn, S.; Lamy, E. The Brassica epithionitrile 1-cyano-2,3-epithiopropane triggers cell death in human liver cancer cells in vitro. Mol. Nutr. Food Res. 2015, 59, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Matusheski, N.V.; Jeffery, E. Comparison of the bioactivity of two glucoraphanin hydrolysis products found in broccoli, sulforaphane and sulforaphane nitrile. J. Agric. Food Chem. 2001, 49, 5743–5749. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Schreiner, M. Isothiocyanates, nitriles, and epithionitriles from glucosinolates are affected by genotype and developmental stage in Brassica oleracea varieties. Front. Plant Sci. 2017, 8, 1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klopsch, R.; Witzel, K.; Börner, A.; Schreiner, M.; Hanschen, F.S. Metabolic profiling of glucosinolates and their hydrolysis products in a germplasm collection of Brassica rapa turnips. Food Res. Int. 2017, 100, 392–403. [Google Scholar] [CrossRef]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; De Schrijver, R.; Hansen, M.; Gerhäuser, C.; Mithen, R.; et al. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2008, 53, S219. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P.; Obregón, S.; Padilla, G.; De Haro, A. Seasonal variation in glucosinolate content in Brassica oleracea crops grown in northwestern Spain. Phytochemistry 2008, 69, 403–410. [Google Scholar] [CrossRef]
- Rosa, E.A.; Rodrigues, A.S. Total and individual glucosinolate content in 11 broccoli cultivars grown in early and late seasons. HortScience 2001, 36, 56–59. [Google Scholar] [CrossRef]
- Nuñez-Gómez, V.; Baenas, N.; Navarro-González, I.; García-Alonso, F.J.; Moreno, D.A.; González-Barrio, R.; Periago, M.J. Seasonal variation of health-promoting bioactives in broccoli and methyl-jasmonate pre-harvest treatments to enhance their contents. Foods 2020, 9, 1371. [Google Scholar] [CrossRef] [PubMed]
- Charron, C.S.; Sams, C.E. Glucosinolate content and myrosinase activity in rapid-cycling Brassica oleracea grown in a controlled environment. J. Am. Soc. Hortic. Sci. 2004, 129, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Mølmann, J.A.; Steindal, A.L.; Bengtsson, G.B.; Seljåsen, R.; Lea, P.; Skaret, J.; Johansen, T.J. Effects of temperature and photoperiod on sensory quality and contents of glucosinolates, flavonols and vitamin C in broccoli florets. Food Chem. 2015, 172, 47–55. [Google Scholar] [CrossRef]
- Pereira, F.M.V.; Rosa, E.; Fahey, J.W.; Stephenson, K.K.; Carvalho, R.; Aires, A. Influence of temperature and ontogeny on the levels of glucosinolates in nroccoli (Brassica oleracea var. italica) sprouts and their effect on the induction of mammalian phase 2 enzymes. J. Agric. Food Chem. 2002, 50, 6239–6244. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S. Domestic boiling and salad preparation habits affect glucosinolate degradation in red cabbage (Brassica oleracea var. capitata f. rubra). Food Chem. 2020, 321, 126694. [Google Scholar] [CrossRef] [PubMed]
- Ciska, E.; Martyniak-Przybyszewska, B.; Kozlowska, H. Content of glucosinolates in cruciferous vegetables grown at the same site for two years under different climatic conditions. J. Agric. Food Chem. 2000, 48, 2862–2867. [Google Scholar] [CrossRef]
- Brown, A.F.; Yousef, G.G.; Jeffery, E.H.; Klein, B.P.; Wallig, M.A.; Kushad, M.M.; Juvik, J.A. Glucosinolate profiles in broccoli: Variation in levels and implications in breeding for cancer chemoprotection. J. Am. Soc. Hortic. Sci. 2002, 127, 807–813. [Google Scholar] [CrossRef]
- Schonhof, I.; Blankenburg, D.; Müller, S.; Krumbein, A. Sulfur and nitrogen supply influence growth, product appearance, and glucosinolate concentration of broccoli. J. Plant Nutr. Soil Sci. 2007, 170, 65–72. [Google Scholar] [CrossRef]
- Omirou, M.D.; Papadopoulou, K.K.; Papastylianou, I.; Constantinou, M.; Karpouzas, D.G.; Asimakopoulos, I.; Ehaliotis, C. Impact of nitrogen and sulfur fertilization on the composition of glucosinolates in relation to sulfur assimilation in different plant organs of broccoli. J. Agric. Food Chem. 2009, 57, 9408–9417. [Google Scholar] [CrossRef]
- Charron, C.S.; Saxton, A.M.; Sams, C.E. Relationship of climate and genotype to seasonal variation in the glucosinolate-myrosinase system. I. Glucosinolate content in ten cultivars of Brassica oleracea grown in fall and spring seasons. J. Sci. Food Agric. 2005, 85, 671–681. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.; García-Viguera, C. Health-promoting compounds in broccoli as influenced by refrigerated transport and retail sale period. J. Agric. Food Chem. 2003, 51, 3029–3034. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Rosa, E.A.S. Effect of post-harvest treatments on the level of glucosinolates in broccoli. J. Sci. Food Agric. 1999, 79, 1028–1032. [Google Scholar] [CrossRef]
- Kang, J.-H.; Woo, H.-J.; Park, J.-B.; Chun, H.H.; Park, C.W.; Bin Song, K. Effect of storage in pallet-unit controlled atmosphere on the quality of Chinese cabbage (Brassica rapa L. spp. pekinensis) used in kimchi manufacturing. LWT 2019, 111, 436–442. [Google Scholar] [CrossRef]
- Osher, Y.; Chalupowicz, D.; Maurer, D.; Ovadia-Sadeh, A.; Lurie, S.; Fallik, E.; Kenigsbuch, D. Summer storage of cabbage. Postharvest Biol. Technol. 2018, 145, 144–150. [Google Scholar] [CrossRef]
- Lucarini, M.; Di Cocco, M.E.; Raguso, V.; Milanetti, F.; Durazzo, A.; Lombardi-Boccia, G.; Santini, A.; Delfini, M.; Sciubba, F. NMR-based metabolomic comparison of Brassica oleracea (var. italica): Organic and conventional farming. Foods 2020, 9, 945. [Google Scholar] [CrossRef] [PubMed]
- Textor, S.; Gershenzon, J. Herbivore induction of the glucosinolate–myrosinase defense system: Major trends, biochemical bases and ecological significance. Phytochem. Rev. 2008, 8, 149–170. [Google Scholar] [CrossRef] [Green Version]
- Hanschen, F.S.; Kaufmann, M.; Kupke, F.; Hackl, T.; Kroh, L.W.; Rohn, S.; Schreiner, M. Brassica vegetables as sources of epithionitriles: Novel secondary products formed during cooking. Food Chem. 2018, 245, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Fechner, J.; Kaufmann, M.; Herz, C.; Eisenschmidt, D.; Lamy, E.; Kroh, L.W.; Hanschen, F.S. The major glucosinolate hydrolysis product in rocket (Eruca sativa L.), sativin, is 1,3-thiazepane-2-thione: Elucidation of structure, bioactivity, and stability compared to other rocket isothiocyanates. Food Chem. 2018, 261, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Shikita, M.; Fahey, J.W.; Golden, T.R.; Holtzclaw, W.D.; Talalay, P. An unusual case of ‘uncompetitive activation’ by ascorbic acid: Purification and kinetic properties of a myrosinase from Raphanus sativus seedlings. Biochem. J. 1999, 341, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Kyung, K.; Fleming, H.; Young, C.; Haney, C. 1-Cyano-2,3-epithiopropane as the primary sinigrin hydrolysis product of fresh cabbage. J. Food Sci. 1995, 60, 157–159. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Swarup, R.; Juvik, J.A.; Mithen, R.; Bennett, M.; Jeffery, E. Epithiospecifier protein from broccoli (Brassica oleracea L. ssp. italica) inhibits formation of the anticancer agent sulforaphane. J. Agric. Food Chem. 2006, 54, 2069–2076. [Google Scholar] [CrossRef]
- Burow, M.; Markert, J.; Gershenzon, J.; Wittstock, U. Comparative biochemical characterization of nitrile-forming proteins from plants and insects that alter myrosinase-catalysed hydrolysis of glucosinolates. FEBS J. 2006, 273, 2432–2446. [Google Scholar] [CrossRef]
- Wittstock, U.; Meier, K.; Dörr, F.; Ravindran, B.M. NSP-dependent simple nitrile formation dominates upon breakdown of major aliphatic glucosinolates in roots, seeds, and seedlings of Arabidopsis thaliana Columbia-0. Front. Plant Sci. 2016, 7, 1821. [Google Scholar] [CrossRef] [Green Version]
- Román, J.; González, D.; Inostroza-Ponta, M.; Mahn, A. Molecular modeling of epithiospecifier and nitrile-specifier proteins of broccoli and their interaction with aglycones. Molecules 2020, 25, 772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witzel, K.; Abu Risha, M.; Albers, P.; Börnke, F.; Hanschen, F.S. Identification and characterization of three epithiospecifier protein isoforms in Brassica oleracea. Front. Plant Sci. 2019, 10, 1552. [Google Scholar] [CrossRef] [PubMed]
- Geilfus, C.-M.; Hasler, K.; Witzel, K.; Gerendás, J.; Mühling, K.H. Interactive effects of genotype and N/S-supply on glucosinolates and glucosinolate breakdown products in Chinese cabbage (Brassica rapa L. ssp. pekinensis). J. Appl. Bot. Food Qual. 2016, 89, 279–286. [Google Scholar]
- Meschede, C.A.C.; Abdalla, M.A.; Mühling, K.H. Sulfur but not nitrogen supply increases the ITC/nitrile ratio in pak choi (Brassica rapa subsp. chinensis (L.) Hanelt). J. Appl. Bot. Food Qual. 2020, 93, 95–104. [Google Scholar]
- Burow, M.; Losansky, A.; Müller, R.; Plock, A.; Kliebenstein, D.J.; Wittstock, U. The genetic basis of constitutive and herbivore-induced ESP-independent nitrile formation in Arabidopsis. Plant Physiol. 2008, 149, 561–574. [Google Scholar] [CrossRef] [Green Version]
- Mumm, R.; Burow, M.; Bukovinszkine’Kiss, G.; Kazantzidou, E.; Wittstock, U.; Dicke, M.; Gershenzon, J. Formation of simple nitriles upon glucosinolate hydrolysis affects direct and indirect defense against the specialist herbivore, Pieris rapae. J. Chem. Ecol. 2008, 34, 1311–1321. [Google Scholar] [CrossRef] [Green Version]
- Jasper, J.; Wagstaff, C.; Bell, L. Growth temperature influences postharvest glucosinolate concentrations and hydrolysis product formation in first and second cuts of rocket salad. Postharvest Biol. Technol. 2020, 163, 111157. [Google Scholar] [CrossRef]
- Ku, K.-M.; Jeffery, E.; Juvik, J.A. Influence of seasonal variation and methyl jasmonate mediated induction of glucosinolate biosynthesis on quinone reductase activity in broccoli florets. J. Agric. Food Chem. 2013, 61, 9623–9631. [Google Scholar] [CrossRef]





| Date of Purchase | |||
|---|---|---|---|
| Supplier | Abbreviation | White Cabbage | Red Cabbage |
| CON1 | S1 | 04.09.2019 | 04.09.2019 |
| S2 | 16.09.2019 | 16.09.2019 | |
| S3 | 30.09.2019 | 30.09.2019 | |
| S4 | 21.10.2019 | 21.10.2019 | |
| S5 | 04.11.2019 | 04.11.2019 | |
| S6 | 18.11.2019 | 18.11.2019 | |
| CON2 | S1 | 05.09.2019 | 05.09.2019 |
| S2 | 16.09.2019 | 16.09.2019 | |
| S3 | 30.09.2019 | 30.09.2019/01.10.2019 | |
| S4 | 21.10.2019 | 21.10.2019/23.10.2019 | |
| S5 | 04.11.2019 | 4.11.2019/06.11.2019 | |
| S6 | 18.11.2019 | 18.11.2019 | |
| ORG1 | S1 | 09.09.2019 | 09.09.2019 |
| S2 | 19.09.2019 | 19.09.2019 | |
| S3 | 01.10.2019 | 01.10.2019 | |
| S4 | 23.10.2019 | 23.10.2019 | |
| S5 | 06.11.2019 | 06.11.2019 | |
| S6 | 20.11.2019 | 20.11.2019 | |
| Fresh harvest from field (IGZ) | Date of harvest | ||
| White Cabbage | Red Cabbage | ||
| 29.10.2019 | 10.10.2019 | ||
| Supplier | Origin of Cultivation | Samples/Date of Harvest (IGZ) | Harvest Season | Cabbage Type | Cabbage Genotype | Soil Type and Field | Fertilizer | Certifica-Tion Mark | Storage Conditions *1 | Storage Conditions *2 |
|---|---|---|---|---|---|---|---|---|---|---|
| CON1 | 25792, Neuenkirchen Schleswig-Holstein | S1–S3 | Summer, autumn | (1), (2) | White cabbage hybrids | Sea marsh | Urea, CAN, phosphate and potash | QS | (i) | 20 °C day/night |
| S1–S4 | Red cabbage hybrids | |||||||||
| CON1 | 25709, Helse Schleswig-Holstein | S4–S6 | Autumn, winter | (2), (3) | White cabbage hybrids | Sea marsh, pH-value 7.0–7.4 | PKS fertilizer (blends), ammonium nitrate and urea, calcium cyanamide | QS | (ii) | 20 °C day/night |
| S5, S6 | Red cabbage hybrids | |||||||||
| CON2 | 25792, Neuenkirchen Schleswig-Holstein | S1–S6 | Summer, autumn, winter | (1), (2), (3) | Red and white cabbage hybrids | Sea marsh | Urea, CAN, phosphate and potash | QS | (iii) | 7–10 °C day/night in cooling counter, max. 1 week |
| ORG1 | 17237, Blankensee Mecklenburg-Western Pomerania | S1, S4, S5 | Autumn | (2) | White cabbage hybrids | Sandy loam | Hair-meal pellets | Bioland | (iv) | 10 °C day/night in cooling counter, max. 1 week |
| S1 | Red cabbage hybrids | |||||||||
| ORG1 | 25761, Hedwigenkoog Schleswig-Holstein | S2, S3 | Autumn | (2) | White cabbage, non-hybrid | Sea Marsh | Compost | Demeter | (iv) | Day/night in cooling counter, max. 1 week |
| S2, S3, S4, S5 | Red cabbage, non-hybrid | |||||||||
| ORG1 | 15306, Vierlinden Brandenburg | S6 | Autumn | (2) | White cabbage hybrids | Sandy loam | Hair-meal pellets | Bioland | (v) | Day/night in cooling counter, max. 1 week |
| ORG1 | 14715, Seeblick Brandenburg | S6 | Autumn | (2) | Red cabbage, non-hybrids | Sandy loam | Compost | Demeter | (iv) | Day/night in cooling counter, max. 1 week |
| IGZ | 14979, Großbeeren Brandenburg | 10 October 2019 | Autumn | (2) | Red cabbage (Redma RZ F1), hybrid | Silty loam | CAN, patentkali | - | No storage | No storage |
| IGZ | 14979, Großbeeren Brandenburg | 29 October 2019 | Autumn | (2) | White cabbage (Dottenfelder Dauer), non-hybrid | Silty loam | Vinasse | - | No storage | No storage |
| Glucosinolates (GLSs) | Corresponding Breakdown Products | |||||||
|---|---|---|---|---|---|---|---|---|
| Isothiocyanate (ITC) | Nitrile | Epithionitrile (ETN) | ||||||
| Structure | Abbreviation | Name (trivial name) | Abbreviation | Name | Abbreviation | Name | Abbreviation | Name |
 | ||||||||
 | Allyl | allyl GLS (sinigrin) | Allyl-ITC | 2-propenyl ITC | Allyl-CN | 3-butenenitrile | CETP | 1-cyano-2,3-epithiopropane |
 | 3But | 3-butenyl GLS (gluconapin) | 3But-ITC | 3-butenyl ITC | 3But-CN | 4-pentenenitrile | CETB | 1-cyano-3,4-epithiobutane |
 | 2OH3But | 2-(R)-2-hydroxy-3-butenyl GLS (progoitrin) | OZT | 5-vinyl-1,3-oxazolidine-2-thione | 3-hydroxy-pentenenitrile | CHETB A CHETB B | 1-cyano-2-hydroxy-3,4-epithiobutane | |
 | 3MSOP | 3-(methylsulfinyl)propyl GLS (glucoiberin) | 3MSOP-ITC | 3-(methylsulfinyl)-propyl ITC | 3MSOP-CN | 4-(methylsulfinyl)-butanenitrile | - | - |
 | 4MSOB | 4-(methylsulfinyl)butyl GLS (glucoraphanin) | 4MSOB-ITC | 4-(methylsulfinyl)butyl ITC | 4MSOB-CN | 5-(methylsulfinyl)-pentanenitrile | - | - |
 | I3M | indol-3-ylmethyl GLS (glucobrassicin) | n.d. | indole-3-acetonitrile | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wermter, N.S.; Rohn, S.; Hanschen, F.S. Seasonal Variation of Glucosinolate Hydrolysis Products in Commercial White and Red Cabbages (Brassica oleracea var. capitata). Foods 2020, 9, 1682. https://doi.org/10.3390/foods9111682
Wermter NS, Rohn S, Hanschen FS. Seasonal Variation of Glucosinolate Hydrolysis Products in Commercial White and Red Cabbages (Brassica oleracea var. capitata). Foods. 2020; 9(11):1682. https://doi.org/10.3390/foods9111682
Chicago/Turabian StyleWermter, Nicole S., Sascha Rohn, and Franziska S. Hanschen. 2020. "Seasonal Variation of Glucosinolate Hydrolysis Products in Commercial White and Red Cabbages (Brassica oleracea var. capitata)" Foods 9, no. 11: 1682. https://doi.org/10.3390/foods9111682