Comparison of the Structural Properties and Nutritional Fraction of Corn Starch Treated with Thermophilic GH13 and GH57 α-Glucan Branching Enzymes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Construction of PhGBE and CbGBE Overexpression Vectors
2.3. Expression and Purification of Recombinant PhGBE and CbGBE
2.4. GBE Activity Assays
2.5. Substrate Specificities of the GBEs
2.6. Determination of the Side Chain Length Distribution
2.7. Preparation of GBE-Modified Starches
2.8. Determination of the Linkage Ratios
2.9. Solid-State 13C NMR with Cross Polarization/Magic Angle Sample Spinning
2.10. X-ray Diffraction Patterns and Relative Crystallinity
2.11. Determination of the Starch Fractions based on Digestibility
2.12. Statistical Analysis
3. Results and Discussion
3.1. Purification of Recombinant PhGBE and CbGBE and Assessment of Their Branching Activity for Various Substrates
3.2. Branch Activities of PhGBE and CbGBE on AM
3.3. Side Chain Length Distribution of GBE-Modified Starches
3.4. Glycosidic Linkage Ratio of GBE-Modified Starches
3.5. 13C CP/MAS NMR and XRD Analysis
3.6. In Vitro Digestibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Englyst, H.N.; Kingman, S.; Cummings, J. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- Englyst, K.N.; Vinoy, S.; Englyst, H.N.; Lang, V. Glycaemic index of cereal products explained by their content of rapidly and slowly available glucose. Brit. J. Nutr. 2003, 89, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Goff, L.; Cowland, D.; Hooper, L.; Frost, G. Low glycaemic index diets and blood lipids: A systematic review and meta-analysis of randomised controlled trials. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.-H.; Yan, L.; Phillips, R.J.; Reuhs, B.L.; Jones, K.; Rose, D.R.; Nichols, B.L.; Quezada-Calvillo, R.; Yoo, S.-H.; Hamaker, B.R. Enzyme-synthesized highly branched maltodextrins have slow glucose generation at the mucosal α-glucosidase level and are slowly digestible in vivo. PLoS ONE 2013, 8, e59745. [Google Scholar] [CrossRef] [PubMed]
- Do, H.V.; Lee, E.-J.; Park, J.-H.; Park, K.-H.; Shim, J.-Y.; Mun, S.; Kim, Y.-R. Structural and physicochemical properties of starch gels prepared from partially modified starches using Thermus aquaticus 4-α-glucanotransferase. Carbohydr. Polym. 2012, 87, 2455–2463. [Google Scholar] [CrossRef]
- Park, C.-S.; Park, I. The structural characteristics of amylosucrase-treated waxy corn starch and relationship between its in vitro digestibility. Food Sci. Biotechnol. 2017, 26, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Miao, M.; Jiang, H.; Xue, J.; Jiang, B.; Zhang, T.; Gao, Y.; Jia, Y. Partial branching enzyme treatment increases the low glycaemic property and α-1, 6 branching ratio of maize starch. Food Chem. 2014, 164, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.I.; Choi, H.J.; Chung, K.M.; Hamaker, B.R.; Park, K.H.; Moon, T.W. Slowly digestible starch from debranched waxy sorghum starch: Preparation and properties. Cereal Chem. 2004, 81, 404–408. [Google Scholar] [CrossRef]
- Preiss, J. Bacterial glycogen synthesis and its regulation. Annu. Rev. Microbiol. 1984, 38, 419–458. [Google Scholar] [CrossRef]
- Henrissat, B.; Bairoch, A. Updating the sequence-based classification of glycosyl hydrolases. Biochem. J. 1996, 316, 695. [Google Scholar] [CrossRef]
- Chiba, S. Molecular mechanism in α-glucosidase and glucoamylase. J. Biosci. Biotechnol. Biochem. 1997, 61, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Kanai, T.; Takata, H.; Kuriki, T.; Imanaka, T. A novel branching enzyme of the GH-57 family in the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J. Bacteriol. 2006, 188, 5915–5924. [Google Scholar] [CrossRef] [PubMed]
- Palomo, M.; Pijning, T.; Booiman, T.; Dobruchowska, J.M.; van der Vlist, J.; Kralj, S.; Planas, A.; Loos, K.; Kamerling, J.P.; Dijkstra, B.W.; et al. Thermus thermophilus glycoside hydrolase family 57 branching enzyme: crystal structure, mechanism of action and products formed. J. Biol. Chem. 2011, 5, 3520–3530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Leemhuis, H.; van der Maarel, M. Synthesis of highly branched α-glucans with different structures using GH13 and GH57 glycogen branching enzymes. Carbohydr. Polym. 2019, 216, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.-T.; Lee, C.-K.; Kim, Y.-W.; Lee, S.-J.; Zhang, R.; Withers, S.G.; Kim, Y.-R.; Auh, J.-H.; Park, K.-H. Amylolytically-resistant tapioca starch modified by combined treatment of branching enzyme and maltogenic amylase. Carbohydr. Polym. 2009, 75, 9–14. [Google Scholar] [CrossRef]
- Li, Y.; Ren, J.; Liu, J.; Sun, L.; Wang, Y.; Liu, B.; Li, C.; Li, Z. Modification by α-d-glucan branching enzyme lowers the in vitro digestibility of starch from different sources. Int. J. Biol. Macromol. 2018, 107, 1758–1764. [Google Scholar] [CrossRef]
- Haki, G.; Rakshit, S. Developments in industrially important thermostable enzymes: A review. Bioresour. Technol. 2003, 89, 17–34. [Google Scholar] [CrossRef]
- Na, S.; Park, M.; Jo, I.; Cha, J.; Ha, N.-C. Structural basis for the transglycosylase activity of a GH57-type glycogen branching enzyme from Pyrococcus horikoshii. Biochem. Biophys. Res. Commun. 2017, 484, 850–856. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Guan, H.P.; Preiss, J. Differentiation of the properties of the branching isozymes from maize (Zea mays). Plant Physiol. 1993, 102, 1269–1273. [Google Scholar] [CrossRef]
- Gidley, M.J.; Bociek, S.M. Molecular organization in starches: A carbon 13 CP/MAS NMR study. J. Am. Chem. Soc. 1985, 107, 7040–7044. [Google Scholar] [CrossRef]
- Nara, S.; Komiya, T. Studies on the relationship between water-satured state and crystallinity by the diffraction method for moistened potato starch. Starch-Stärke 1983, 35, 407–410. [Google Scholar] [CrossRef]
- Sawada, T.; Nakamura, Y.; Ohdan, T.; Saitoh, A.; Francisco, P.B., Jr.; Suzuki, E.; Fujita, N.; Shimonaga, T.; Fujiwara, S.; Tsuzuki, M. Diversity of reaction characteristics of glucan branching enzymes and the fine structure of α-glucan from various sources. Arch. Biochem. Biophys. 2014, 562, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Kittisuban, P.; Lee, B.-H.; Suphantharika, M.; Hamaker, B.R. Slow glucose release property of enzyme-synthesized highly branched maltodextrins differs among starch sources. Carbohydr. Polym. 2014, 107, 182–191. [Google Scholar] [CrossRef]
- Gidley, M.J.; Bulpin, P.V. Crystallisation of malto-oligosaccharides as models of the crystalline forms of starch: Minimum chain-length requirement for the formation of double helices. Carbohydr. Res. 1987, 161, 291–300. [Google Scholar] [CrossRef]
- Hizukuri, S.; Takeda, Y.; Usami, S.; Takase, Y. Effect of aliphatic hydrocarbon groups on the crystallization of amylodextrin: Model experiments for starch crystallization. Carbohydr. Res. 1980, 83, 193–199. [Google Scholar] [CrossRef]
- Jo, A.R.; Kim, H.R.; Choi, S.J.; Lee, J.S.; Chung, M.N.; Han, S.K.; Park, C.-S.; Moon, T.W. Preparation of slowly digestible sweet potato Daeyumi starch by dual enzyme modification. Carbohydr. Polym. 2016, 143, 164–171. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Venkatachalam, M.; Hamaker, B.R. Structural basis for the slow digestion property of native cereal starches. Biomacromolecules 2006, 7, 3259–3266. [Google Scholar] [CrossRef]
- Lee, C.-K.; Le, Q.-T.; Kim, Y.-H.; Shim, J.-H.; Lee, S.-J.; Park, J.-H.; Lee, K.-P.; Song, S.-H.; Auh, J.H.; Lee, S.-J. Enzymatic synthesis and properties of highly branched rice starch amylose and amylopectin cluster. J. Agric. Food Chem. 2007, 56, 126–131. [Google Scholar] [CrossRef]
- Miao, M.; Li, R.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z. Structure and physicochemical properties of octenyl succinic esters of sugary maize soluble starch and waxy maize starch. Food Chem. 2014, 151, 154–160. [Google Scholar] [CrossRef]
- Takii, H.; Ishihara, K.; Kometani, T.; Okada, S.; Fushiki, T. Enhancement of swimming endurance in mice by highly branched cyclic dextrin. Biosci. Biotechnol. Biochem. 1999, 63, 2045–2052. [Google Scholar] [CrossRef] [PubMed]




| Starch | Relative Peak Area (%) | ||||
|---|---|---|---|---|---|
| DP 6−9 (A Chains) | DP 10−12 (A Chains) | DP 13−24 (B1 Chains) | DP 25−36 (B2 Chains) | ≥DP 37 (B3 and Longer) | |
| control 1 | 8.1 ± 0.2 c2,3 | 14.7 ± 0.4 b | 56.0 ± 0.7 a | 15.9 ± 0.7 a | 5.1 ± 0.1 a |
| PhGBE | 10.0 ± 0.4 b | 14.9 ± 0.2 b | 54.3 ± 0.4 b | 15.3 ± 0.6 a | 4.9 ± 0.5 a |
| CbGBE | 25.2 ± 0.5 a | 20.9 ± 0.1 a | 42.1 ± 1.1 c | 7.8 ± 1.1 b | 2.1 ± 0.7 b |
| Starches | α-1,4 Linkage (%) | α-1,6 Linkage (%) |
|---|---|---|
| Native | 97.2 ± 0.1 a1,2 | 2.8 ± 0.1 c |
| Control | 97.2 ± 0.1 a | 2.8 ± 0.1 c |
| PhGBE-treated | 95.6 ± 0.1 b | 4.4 ± 0.1 b |
| CbGBE-treated | 91.4 ± 0.1 c | 8.6 ± 0.1 a |
| Starches | XRD Crystallinity (%) | 13C CP/MAS NMR | Double Helix (NMR)–Crystallinity (XRD) | |
|---|---|---|---|---|
| Double Helix (%) | Amorphous (%) | |||
| Native | 42.9 ± 0.1 1 | 42.1 ± 0.1 a3 | 57.9 ± 0.0 c | N/A |
| Control | N/A 2 | 23.3 ± 0.0 d | 76.7 ± 0.1 a | 23.3 |
| PhGBE-treated | N/A | 29.1 ± 0.1 bc | 70.9 ± 0.1 b | 29.1 |
| CbGBE-treated | N/A | 30.4 ± 0.0 b | 69.6 ± 0.0 b | 30.4 |
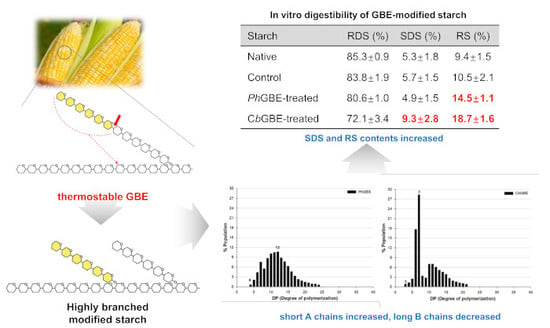
| Starches | RDS (%) | SDS (%) | RS (%) | SDS + RS |
|---|---|---|---|---|
| Native | 85.3 ± 0.9 a1,2 | 5.3 ± 1.8 c | 9.4 ± 1.5 d | 14.7 |
| Control | 83.8 ± 1.9 a | 5.7 ± 1.5 c | 10.5 ± 2.1 c | 16.2 |
| PhGBE-treated | 80.6 ± 1.0 b | 4.9 ± 1.5 b | 14.5 ± 1.1 b | 19.4 |
| CbGBE-treated | 72.1 ± 3.4 c | 9.3 ± 2.8 a | 18.7 ± 1.6 a | 28.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, I.; Park, M.; Yoon, N.; Cha, J. Comparison of the Structural Properties and Nutritional Fraction of Corn Starch Treated with Thermophilic GH13 and GH57 α-Glucan Branching Enzymes. Foods 2019, 8, 452. https://doi.org/10.3390/foods8100452
Park I, Park M, Yoon N, Cha J. Comparison of the Structural Properties and Nutritional Fraction of Corn Starch Treated with Thermophilic GH13 and GH57 α-Glucan Branching Enzymes. Foods. 2019; 8(10):452. https://doi.org/10.3390/foods8100452
Chicago/Turabian StylePark, Inmyoung, Minjeong Park, Naeun Yoon, and Jaeho Cha. 2019. "Comparison of the Structural Properties and Nutritional Fraction of Corn Starch Treated with Thermophilic GH13 and GH57 α-Glucan Branching Enzymes" Foods 8, no. 10: 452. https://doi.org/10.3390/foods8100452