Films Based on Thermoplastic Starch Blended with Pine Resin Derivatives for Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. TPS-Resin Blends
2.2.2. Dynamic Mechanical Thermal Analysis
2.2.3. Water Uptake
2.2.4. Surface Characterization, Color, and Wettability
2.2.5. X-ray Diffraction (XDR)
2.2.6. Disintegration under Composting Conditions
3. Results
3.1. Dynamic Mechanical Thermal Analysis
3.2. Surface Characterization, Color, and Wettability
3.3. Water Uptake
3.4. X-ray Diffraction Pattern (XDR)
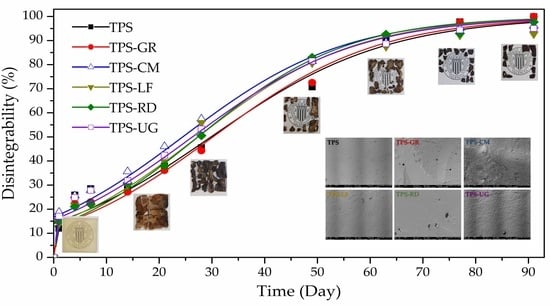
3.5. Disintegration under Composting Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.; Chen, E.Y.-X. Future Directions for Sustainable Polymers. Trends Chem. 2019, 1, 148–151. [Google Scholar] [CrossRef]
- Schneiderman, D.K.; Hillmyer, M.A. 50th Anniversary Perspective: There Is a Great Future in Sustainable Polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Gkartzou, E.; Koumoulos, E.P.; Charitidis, C.A. Production and 3D printing processing of bio-based thermoplastic filament. Manuf. Rev. 2017, 4, 1–14. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.-X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Plastics Europe Market Research Group (PEMRG). Plastics-The Facts 2019 an Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope: Brussels, Belgium, 2019. [Google Scholar]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Ganewatta, M.S.; Tang, C. Sustainable polymers from biomass: Bridging chemistry with materials and processing. Prog. Polym. Sci. 2020, 101, 101197–101211. [Google Scholar] [CrossRef]
- Pillai, C.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peltzer, M.A.; López, J.; Garrigós, M.D.C.; Valente, A.J.; Jiménez, A. Functional properties of sodium and calcium caseinate antimicrobial active films containing carvacrol. J. Food Eng. 2014, 121, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Galbis, J.A.; De Gracia García-Martín, M.; Violante De Paz, M.; Galbis, E.; García-Martín, M.D.G.; De Paz, M.V.; Galbis, E. Synthetic Polymers from Sugar-Based Monomers; American Chemical Society: Washington, DC, USA, 2020; Volume 116, pp. 1600–1636. [Google Scholar]
- Papageorgiou, G.Z. Thinking Green: Sustainable Polymers from Renewable Resources. Polymers 2018, 10, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gu, Y.; Castellarin, S.D.; Kitts, D.D.; Pratap-Singh, A. Development and Characterization of the Edible Packaging Films Incorporated with Blueberry Pomace. Foods 2020, 9, 1599. [Google Scholar] [CrossRef]
- Díaz-Galindo, E.P.; Nesic, A.; Bautista-Baños, S.; García, O.D.; Cabrera-Barjas, G. Corn-Starch-Based Materials Incorporated with Cinnamon Oil Emulsion: Physico-Chemical Characterization and Biological Activity. Foods 2020, 9, 475. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashimi, G.A.; Ammar, A.B.; Lakshmanan, G.; Cacciola, F.; Lakhssassi, N. Development of a Millet Starch Edible Film Containing Clove Essential Oil. Foods 2020, 9, 184. [Google Scholar] [CrossRef] [Green Version]
- Leon-Bejarano, M.; Durmus, Y.; Ovando-Martínez, M.; Simsek, S. Physical, Barrier, Mechanical, and Biodegradability Properties of Modified Starch Films with Nut by-Products Extracts. Foods 2020, 9, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldas, M.; Pavon, C.; López-Martínez, J.; Arrieta, M.P. Pine Resin Derivatives as Sustainable Additives to Improve the Mechanical and Thermal Properties of Injected Moulded Thermoplastic Starch. Appl. Sci. 2020, 10, 2561. [Google Scholar] [CrossRef] [Green Version]
- Aldas, M.; Ferri, J.M.; Lopez-Martinez, J.; Samper, M.D.; Arrieta, M.P. Effect of pine resin derivatives on the structural, thermal, and mechanical properties of Mater-Bi type bioplastic. J. Appl. Polym. Sci. 2020, 137, 48236–48250. [Google Scholar] [CrossRef]
- Angellier, H.; Molina-Boisseau, S.; Dole, P.; Dufresne, A. Thermoplastic Starch-Waxy Maize Starch Nanocrystals Nanocomposites. Biomacromolecules 2006, 7, 531–539. [Google Scholar] [CrossRef]
- Espigulé, E.; Puigvert, X.; Vilaseca, F.; Méndez, J.A.; Mutjé, P.; Gironès, J. Thermoplastic Starch-based Composites Reinforced with Rape Fibers: Water Uptake and Thermomechanical Properties. BioResources 2013, 8, 2620–2630. [Google Scholar] [CrossRef] [Green Version]
- Sessini, V.; Arrieta, M.P.; Raquez, J.M.; Dubois, P.; Kenny, J.M.; Peponi, L. Thermal and composting degradation of EVA/Thermoplastic starch blends and their nanocomposites. Polym. Degrad. Stab. 2019, 159, 184–198. [Google Scholar] [CrossRef]
- Sessini, V.; Arrieta, M.P.; Fernández-Torres, A.; Peponi, L. Humidity-activated shape memory effect on plasticized starch-based biomaterials. Carbohydr. Polym. 2018, 179, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, F.; Aldas, M.; Parres, F.; López-Martínez, J.; Arrieta, M.P. Silane-Functionalized Sheep Wool Fibers from Dairy Industry Waste for the Development of Plasticized PLA Composites with Maleinized Linseed Oil for Injection-Molded Parts. Polymers 2020, 12, 2523. [Google Scholar] [CrossRef] [PubMed]
- Pavon, C.; Aldas, M.; de la Rosa-Ramírez, H.; López-Martínez, J.; Arrieta, M.P. Improvement of PBAT Processability and Mechanical Performance by Blending with Pine Resin Derivatives for Injection Moulding Rigid Packaging with Enhanced Hydrophobicity. Polymers 2020, 12, 2891. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa-Ramirez, H.; Aldas, M.; Ferri, J.M.; López-Martínez, J.; Samper, M.D. Modification of poly (lactic acid) through the incorporation of gum rosin and gum rosin derivative: Mechanical performance and hydrophobicity. J. Appl. Polym. Sci. 2020, 137, 49346–49361. [Google Scholar] [CrossRef]
- Moustafa, H.; El Kissi, N.; Abou-Kandil, A.I.; Abdel-Aziz, M.S.; Dufresne, A.; El Kissi, N.; Abou-Kandil, A.I.; Abdel-Aziz, M.S.; Dufresne, A. PLA/PBAT Bionanocomposites with Antimicrobial Natural Rosin for Green Packaging. ACS Appl. Mater. Interfaces 2017, 9, 20132–20141. [Google Scholar] [CrossRef]
- Langenheim, J. Plant Resins. Am. Sci. 1990, 78, 16–24. [Google Scholar]
- Arrieta, M.P.; Samper, M.; Jiménez-López, M.; Aldas, M.; López, J. Combined effect of linseed oil and gum rosin as natural additives for PVC. Ind. Crop. Prod. 2017, 99, 196–204. [Google Scholar] [CrossRef]
- Rodríguez-García, A.; Martín, J.A.; López, R.; Sanz, A.; Gil, L. Effect of four tapping methods on anatomical traits and resin yield in Maritime pine (Pinus pinaster Ait.). Ind. Crop. Prod. 2016, 86, 143–154. [Google Scholar] [CrossRef]
- Yadav, B.K.; Gidwani, B.; Vyas, A. Rosin: Recent advances and potential applications in novel drug delivery system. J. Bioact. Compat. Polym. 2015, 31, 111–126. [Google Scholar] [CrossRef]
- Chang, R.; Rohindra, D.; Lata, R.; Kuboyama, K.; Ougizawa, T. Development of poly(ε-caprolactone)/pine resin blends: Study of thermal, mechanical, and antimicrobial properties. Polym. Eng. Sci. 2019, 59, 32–41. [Google Scholar] [CrossRef]
- Yao, K.; Tang, C. Controlled Polymerization of Next-Generation Renewable Monomers and Beyond. Macromolecules 2013, 46, 1689–1712. [Google Scholar] [CrossRef]
- Wilbon, P.A.; Chu, F.; Tang, C. Progress in Renewable Polymers from Natural Terpenes, Terpenoids, and Rosin. Macromol. Rapid Commun. 2013, 34, 8–37. [Google Scholar] [CrossRef]
- Aldas, M.; Rayón, E.; López-Martínez, J.; Arrieta, M.P. A Deeper Microscopic Study of the Interaction between Gum Rosin Derivatives and a Mater-Bi Type Bioplastic. Polymers 2020, 12, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavon, C.; Aldas, M.; López-Martínez, J.; Ferrándiz, S. New Materials for 3D-Printing Based on Polycaprolactone with Gum Rosin and Beeswax as Additives. Polymers 2020, 12, 334. [Google Scholar] [CrossRef] [Green Version]
- Sessini, V.; Arrieta, M.P.; Kenny, J.M.; Peponi, L. Processing of edible films based on nanoreinforced gelatinized starch. Polym. Degrad. Stab. 2016, 132, 157–168. [Google Scholar] [CrossRef]
- International Standards Organization. ISO 62:2008 Plastics—Determination of Water Absorption; ISO (International Organization for Standardization): Geneva, Switzerland, 2008. [Google Scholar]
- Liu, G.; Wu, G.; Chen, J.; Kong, Z. Synthesis, modification and properties of rosin-based non-isocyanate polyurethanes coatings. Prog. Org. Coat. 2016, 101, 461–467. [Google Scholar] [CrossRef]
- Atodiresei, G.-V.; Sandu, G.; Tulbure, E.-A.; Vasilache, V.; Butnaru, R. Chromatic Characterization in CieLab System for Natural Dyed Materials, Prior Activation in Atmospheric Plasma Type DBD. Rev. Chim. 2013, 64, 165–169. [Google Scholar]
- Fombuena, V.; Balart, J.; Boronat, T.; Sánchez-Nácher, L.; Garcia-Sanoguera, D. Improving mechanical performance of thermoplastic adhesion joints by atmospheric plasma. Mater. Des. 2013, 47, 49–56. [Google Scholar] [CrossRef]
- International Standards Organization. ISO 20200 Plastics—Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting Conditions in a Laboratory-Scale Test; ISO (International Organization for Standardization): Geneva, Switzerland, 2016. [Google Scholar]
- Arrieta, M.; López, J.; Rayón, E.; Jiménez, A. Disintegrability under composting conditions of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2014, 108, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Fortunati, E.; Puglia, D.; Santulli, C.; Sarasini, F.; Kenny, J.M. Biodegradation of Phormium Tenax/Poly (Lactic Acid) Composites. J. Appl. Polym. Sci. 2012, 125, 562–572. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; López, J.; Kenny, J.M. Bionanocomposite films based on plasticized PLA–PHB/cellulose nanocrystal blends. Carbohydr. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/Organoclay Bionanocomposites Impregnated with Thymol and Cinnamaldehyde by Supercritical Impregnation for Active and Sustainable Food Packaging. Compos. Part B Eng. 2019, 176, 107336–107348. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Parres, F.; López, J.; Jiménez, A. Development of a novel pyrolysis-gas chromatography/mass spectrometry method for the analysis of poly(lactic acid) thermal degradation products. J. Anal. Appl. Pyrolysis 2013, 101, 150–155. [Google Scholar] [CrossRef]
- Da Róz, A.; Carvalho, A.; Gandini, A.; Curvelo, A. The effect of plasticizers on thermoplastic starch compositions obtained by melt processing. Carbohydr. Polym. 2006, 63, 417–424. [Google Scholar] [CrossRef]
- Taguet, A.; Huneault, M.A.; Favis, B.D. Interface/morphology relationships in polymer blends with thermoplastic starch. Polymer 2009, 50, 5733–5743. [Google Scholar] [CrossRef]
- Avérous, L.; Fringant, C.; Moro, L. Plasticized starch–cellulose interactions in polysaccharide composites. Polymer 2001, 42, 6565–6572. [Google Scholar] [CrossRef]
- De Graaf, R.A.; Karman, A.P.; Janssen, L.P.B.M. Material Properties and Glass Transition Temperatures of Different Thermoplastic Starches after Extrusion Processing. Starch-Staerke 2003, 55, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Forssell, P.M.; Mikkilä, J.M.; Moates, G.K.; Parker, R. Phase and glass transition behaviour of concentrated barley starch-glycerol-water mixtures, a model for thermoplastic starch. Carbohydr. Polym. 1997, 34, 275–282. [Google Scholar] [CrossRef]
- Patidar, D.; Agrawal, S.; Saxena, N. Storage modulus and glass transition behaviour of CdS/PMMA nanocomposites. J. Exp. Nanosci. 2011, 6, 441–449. [Google Scholar] [CrossRef]
- Chartoff, R.P.; Sircar, A. Thermal Analysis of Polymers. Encycl. Polym. Sci. Technol. 2004, 1–43. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Long, Z.; Wang, S.; Zhang, J.; Wang, L. Preparation and Performance of Thermoplastic Starch and Microcrystalline Cellulose for Packaging Composites: Extrusion and Hot Pressing. Int. J. Biol. Macromol. 2020, 165, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.P.; Dufresne, A. Plasticized Waxy Maize Starch: Effect of Polyols and Relative Humidity on Material Properties. Biomacromolecules 2002, 3, 1101–1108. [Google Scholar] [CrossRef]
- Edlund, U.; Albertsson, A.-C. Degradable Polymer Microspheres for Controlled Drug Delivery. Polym. Phys. 2007, 157, 67–112. [Google Scholar]
- Ghanbari, A.; Tabarsa, T.; Ashori, A.; Shakeri, A.; Mashkour, M. Preparation and characterization of thermoplastic starch and cellulose nanofibers as green nanocomposites: Extrusion processing. Int. J. Biol. Macromol. 2018, 112, 442–447. [Google Scholar] [CrossRef]
- Mendes, J.; Paschoalin, R.T.; Carmona, V.; Neto, A.R.S.; Marques, A.; Marconcini, J.; Mattoso, L.; Medeiros, E.; Oliveira, J. Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr. Polym. 2016, 137, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Huneault, M.A.; Li, H. Preparation and properties of extruded thermoplastic starch/polymer blends. J. Appl. Polym. Sci. 2012, 126, 96–108. [Google Scholar] [CrossRef]
- Zhao, Q.; Tian, H.; Chen, L.; Zeng, M.; Qin, F.; Wang, Z.; He, Z.; Chen, J. Interactions between soluble soybean polysaccharide and starch during the gelatinization and retrogradation: Effects of selected starch varieties. Food Hydrocoll. 2021, 118, 106765–106779. [Google Scholar] [CrossRef]
- Ochoa-Yepes, O.; Medina-Jaramillo, C.; Guz, L.; Famá, L. Biodegradable and Edible Starch Composites with Fiber-Rich Lentil Flour to Use as Food Packaging. Starch-Staerke 2018, 70, 1700222–1700230. [Google Scholar] [CrossRef]







| WCA | L* | a* | b* | ΔE | YI | |
|---|---|---|---|---|---|---|
| TPS | 53.0 ± 3.0 a | 37.88 ± 0.91 a | −1.47 ± 0.06 a | 5.93 ± 0.15 a | 0.00 ± 0.00 a | 21.04 ± 0.57 a |
| TPS-GR | 80.9 ± 2.9 b | 58.11 ± 1.05 b | −1.72 ± 0.08 b | 17.55 ± 0.68 b | 23.33 ± 1.19 b | 44.6 ± 1.17 b |
| TPS-CM | 70.5 ± 1.9 c | 64.34 ± 0.70 c | −2.81 ± 0.16 c | 23.68 ± 0.80 c | 31.90 ± 1.01 c | 53.47 ± 1.26 c |
| TPS-LF | 86.4 ± 2.9 d | 63.95 ± 0.47 c | −3.64 ± 0.10 d | 12.78 ± 0.43 d | 27.04 ± 0.54 d | 29.35 ± 0.91 d |
| TPS-RD | 77.9 ± 1.7 b | 58.89 ± 0.78 b | −1.16 ± 0.18 e | 20.85 ± 0.60 e | 25.77 ± 0.90 d | 51.89 ± 1.13 d |
| TPS-UG | 86.0 ± 0.9 d | 68.00 ± 0.94 d | −2.07 ± 0.09 f | 17.44 ± 0.49 b | 32.26 ± 0.97 e | 39.46 ± 0.87 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavon, C.; Aldas, M.; López-Martínez, J.; Hernández-Fernández, J.; Arrieta, M.P. Films Based on Thermoplastic Starch Blended with Pine Resin Derivatives for Food Packaging. Foods 2021, 10, 1171. https://doi.org/10.3390/foods10061171
Pavon C, Aldas M, López-Martínez J, Hernández-Fernández J, Arrieta MP. Films Based on Thermoplastic Starch Blended with Pine Resin Derivatives for Food Packaging. Foods. 2021; 10(6):1171. https://doi.org/10.3390/foods10061171
Chicago/Turabian StylePavon, Cristina, Miguel Aldas, Juan López-Martínez, Joaquín Hernández-Fernández, and Marina Patricia Arrieta. 2021. "Films Based on Thermoplastic Starch Blended with Pine Resin Derivatives for Food Packaging" Foods 10, no. 6: 1171. https://doi.org/10.3390/foods10061171