Infection Levels of the Microsporidium Larssoniella duplicati in Populations of the Invasive Bark Beetle Ips duplicatus: From Native to New Outbreak Areas
Abstract
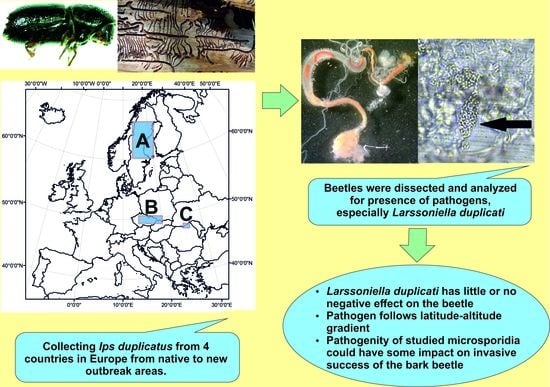
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peltanová, A.; Petrusek, A.; Kment, P.; Juřičková, L. A fast snail’s pace: Colonization of Central Europe by Mediterranean gastropods. Biol. Invasions 2012, 14, 759–764. [Google Scholar] [CrossRef]
- Horák, J.; Hui, C.; Roura-Pascual, N.; Romportl, D. Changing roles of propagule, climate, and land use during extralimital colonization of a rose chafer beetle. Naturwissenschaften 2013, 100, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Nováková, P.; Holuša, J.; Horák, J. The role of geography and host abundance in the distribution of parasitoids of an alien pest. PeerJ 2016, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cudmore, T.J.; Björklund, N.; Carroll, A.L.; Staffan Lindgren, B. Climate change and range expansion of an aggressive bark beetle: Evidence of higher beetle reproduction in native host tree populations. J. Appl. Ecol. 2010, 47, 1036–1043. [Google Scholar] [CrossRef]
- Horák, J.; Brestovanská, T.; Mladenović, S.; Kout, J.; Bogusch, P.; Halda, J.P.; Zasadil, P. Green desert?: Biodiversity patterns in forest plantations. For. Ecol. Manag. 2019, 433, 343–348. [Google Scholar] [CrossRef]
- Hilszczański, J.; Jaworski, T.; Plewa, R.; Horák, J. Tree species and position matter: the role of pests for survival of other insects. Agr. For. Entomol. 2016, 18, 340–348. [Google Scholar] [CrossRef]
- Lukášová, K.; Holuša, J. Invazní Druhy Hmyzu na Lesních Dřevinách, 1st ed.; Czech University of Life Sciences in Prague: Prague, Czech Republic, 2015; ISBN 978-80-213-2606-4. [Google Scholar]
- Pfeffer, A. Kůrovci—Scolytidae (Řád: Brouci—Coleoptera), 6th ed.; Fauna ČSR, Nakladatelství československé akademie věd: Prague, Czech Republic, 1955. [Google Scholar]
- Jönsson, A.M.; Schroeder, L.M.; Lagergren, F.; Anderbrant, O.; Smith, B. Guess the impact of Ips typographus-An ecosystem modelling approach for simulating spruce bark beetle outbreaks. Agric. For. Meteorol. 2012, 166–167, 188–200. [Google Scholar] [CrossRef]
- Seidl, R.; Schelhaas, M.J.; Lindner, M.; Lexer, M.J. Modelling bark beetle disturbances in a large scale forest scenario model to assess climate change impacts and evaluate adaptive management strategies. Reg. Environ. Chang. 2009, 9, 101–119. [Google Scholar] [CrossRef]
- Schelhaas, M.J.; Hengeveld, G.; Moriondo, M.; Reinds, G.J.; Kundzewicz, Z.W.; ter Maat, H.; Bindi, M. Assessing risk and adaptation options to fires and windstorms in European forestry. Mitig. Adapt. Strateg. Glob. Chang. 2010, 15, 681–701. [Google Scholar] [CrossRef] [Green Version]
- Holuša, J.; Zahradník, P.; Knížek, M.; Drápela, K. Seasonal flight activity of the double-spined spruce bark-beetle Ips duplicatus (Coleoptera, Curculionidae, Scolytinae) in Silesia (Czech Republic). Biologia 2003, 58, 935–941. [Google Scholar]
- Fiala, T.; Holuša, J. Occurrence of the Invasive Bark Beetle Phloeosinus aubei on Common Juniper Trees in the Czech Republic. Forests 2018, 10, 12. [Google Scholar] [CrossRef]
- Bentz, B.J.; Régniére, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.F.; Seybold, S.J. Climate Change and Bark Beetles of the Western United States and Canada: Direct and Indirect Effects. Bioscience 2010, 60, 602–613. [Google Scholar] [CrossRef] [Green Version]
- Grodzki, W. Mozliwosci kontroli liczebnosci populacji kornika zroslozebnego Ips duplicatus C.R.Sahlb. na poludniu Polski. Sylwan 1997, 11, 25–36. [Google Scholar]
- Grodzki, W. Distribution range of the double spined bark beetle Ips duplicatus CR Sahlb (Col.: Scolytidae) in the mountain areas of southern Poland. Sylwan 2003, 8, 29–36. [Google Scholar]
- Knížek, M.; Zahradník, P. Mass outbreak of Ips duplicatus Sahlberg (Coleoptera, Scolytidae). In Proceedings of the XX. International Congress of Entomology, Firenze, Italy, 25–31 August 1996; p. 527. [Google Scholar]
- Holuša, J.; Lubojacký, J.; Čurn, V.; Tonka, T.; Lukášová, K.; Horák, J. Combined effects of drought stress and Armillaria infection on tree mortality in Norway spruce plantations. For. Ecol. Manag. 2018, 427, 434–445. [Google Scholar] [CrossRef]
- Boratyński, A. O dysjunkcjach w zasiegu świerka (About disjunctions in the Norway spruce range). In Boratyński, A., Bugala, W., Eds.; Instytut Dendrologii, Bogucki Wydawnictwo Naukowe: Poznań, Poland, 1998; pp. 80–90. ISBN 8386001488. [Google Scholar]
- Holuša, J.; Lubojacký, J.; Knížek, M. Distribution of the double-spined spruce bark beetle Ips duplicatus in the Czech Republic: spreading in 1997–2009. Phytoparasitica 2010, 38, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Duduman, M.-L.; Isaia, G.; Olenici, N. Ips duplicatus (Sahlberg) (Coleoptera: Curculionidae, Scolytinae) distribution in Romania. Bull. Transylv. Univ. Braşov. 2011, 4, 19–26. [Google Scholar]
- Lukášová, K.; Holuša, J. New data on the host specificity of Larssoniella duplicati. Period. Biol. 2013, 115, 455–457. [Google Scholar]
- Holuša, J.; Weiser, J.; Žižka, Z. Pathogens of the spruce bark beetles Ips typographus and Ips duplicatus. Cent. Eur. J. Biol. 2009, 4, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Weiser, J.; Holuša, J.; Žižka, Z. Larssoniella duplicati n.sp. (Microsporidia, Unikaryonidae), a newly described pathogen infecting the double-spined spruce bark beetle, Ips duplicatus (Coleoptera, Scolytidae) in the Czech Republic. J. Pest Sci. 2006, 79, 127–135. [Google Scholar] [CrossRef]
- Wegensteiner, R.; Weiser, J. Annual variation of pathogen occurrence and pathogen prevalence in Ips typographus (Coleoptera, Scolytidae) from the BOKU University Forest Demonstration Centre. J. Pest Sci. 2004, 77, 221–228. [Google Scholar] [CrossRef]
- Takov, D.; Pilarska, D.; Wegensteiner, R. List of Protozoan and Microsporidian Pathogens of Economically Important Bark Beetle Species (Coleoptera: Curculionidae: Scolytinae) in Europe. Acta Zool. Bulg. 2010, 62, 201–209. [Google Scholar]
- Lukášová, K.; Holuša, J. Patogeny Lýkožroutů Rodu Ips (Coleoptera: Curculionidae: Scolytinae): Review. Zprávy Lesn. výzkumu 2012, 57, 230–240. [Google Scholar]
- Pernek, M.; Matošević, D.; Hrašovec, B.; Kučinić, M.; Wegensteiner, R. Occurrence of pathogens in outbreak populations of Pityokteines spp. (Coleoptera, Curculionidae, Scolytinae) in silver fir forests. J. Pest Sci. 2009, 82, 343–349. [Google Scholar] [CrossRef]
- Goertz, D.; Pernek, M.; Haendel, U.; Kohlmayr, B.; Wegensteiner, R. Infection, course of disease and effects of Canningia tomici in Tomicus piniperda and Tomicus minor (Coleoptera: Curculionidae). Period. Biol. 2017, 119, 285–293. [Google Scholar] [CrossRef]
- Duduman, M.L. Field response of the northern spruce bark beetle Ips duplicatus (Sahlberg) (Coleoptera: Curculionidae, Scolytinae) to different combinations of synthetic pheromone with (-)-α-pinene and (+)-limonene. Agric. For. Entomol. 2014, 16, 102–109. [Google Scholar] [CrossRef]
- Byers, J.A.; Schlyter, F.; Birgersson, G.; Francke, W. E-myrcenol in Ips duplicatus: An aggregation pheromone component new for bark beetles. Experientia 1990, 46, 1209–1211. [Google Scholar] [CrossRef]
- Brus, D.J.; Hengeveld, G.M.; Walvoort, D.J.J.; Goedhart, P.W.; Heidema, A.H.; Nabuurs, G.J.; Gunia, K. Statistical mapping of tree species over Europe. Eur. J. For. Res. 2012, 131, 145–157. [Google Scholar] [CrossRef]
- Rangel, T.F.; Diniz-Filho, J.A.F.; Bini, L.M. SAM: A comprehensive application for Spatial Analysis in Macroecology. Ecography 2010, 33, 46–50. [Google Scholar] [CrossRef]
- Holuša, J.; Weiser, J.; Drápela, K. Pathogens of Ips duplicatus (Coleoptera, Scolytidae) in three areas in Central Europe. Acta Protozool. 2007, 46, 157–167. [Google Scholar]
- Holuša, J.; Liška, J. Hypotéza chřadnutí a odumírání smrkových porostů ve Slezsku (Česká Republika). Zprávy Lesn. výzkumu 2002, 47, 9–15. [Google Scholar]
- Lukášová, K.; Holuša, J.; Turčáni, M. Pathogens of Ips amitinus: New species and comparison with Ips typographus. J. Appl. Entomol. 2013, 137, 188–196. [Google Scholar] [CrossRef]
- Holuša, J.; Lukášová, K. Pathogen’s level and parasitism rate in Ips typographus at high population densities: importance of time. J. Appl. Entomol. 2017, 141, 768–779. [Google Scholar] [CrossRef]
- Jurc, M. Insect pathogens with special reference to pathogens of bark beetles (Col., Scolytidae: Ips typographus L.). Preliminary results of isolation of entomopathogenic fungi from two spruce bark beetles in Slovenia. Zb. Gozd. Lesar. 2004, 74, 97–124. [Google Scholar]
- Lukášová, K.; Holuša, J. Gregarina typographi (Eugregarinorida: Gregarinidae) in the Bark Beetle Ips typographus (Coleoptera: Curculionidae): Changes in Infection Level in the Breeding System. Acta Protozool. 2011, 50, 311–318. [Google Scholar] [CrossRef]
- Schnaider, Z.; Sierpinski, Z. Z biologii kornika zrosłozębnego (Ips duplicatus Sahlb.). Rocz. Nauk Lesn. 1955, 13, 437–447. [Google Scholar]
- Holuša, J.; Voigtová, P.; Kula, E.; Křístek, S. Výskyt lýkožrouta severského (Ips duplicatus Sahlberg, 1836) (Coleoptera: Scolitidae) na LS Bruntál LČR, s.p., v roce 2004–2005. Zpr. Ochr. Lesa 2006, 13, 1–46. [Google Scholar]
- Grodzki, W. An attempt to establish the extent and prevalence of the bark beetle Ips duplicatus C.R. Sahlb. (Coleoptera: Scolytidae) in mountain spruce stands in the Western Carpathians. Sylwan 2002, 146, 45–52. [Google Scholar]
- Vakula, J.; Gubka, A.; Zúbrik, M.; Kunca, A. New Methods of Protecting Forests from Double-Spined Spruce Bark Beetle and Other Invasive Species, 1st ed.; National Forest Centre: Zvolen, Slovakia, 2011. [Google Scholar]
- Holuša, J.; Lukášová, K.; Trombik, J. The first record of Ips duplicatus (Coleoptera: Curculionidae, Scolytinae) infestations in central European inner mountains. Sci. Agric. Bohem. 2013, 44, 97–101. [Google Scholar] [CrossRef]
- Wegensteiner, R.; Weiser, J.; Führer, E. Observations on the occurence of pathogens in the bark beatle Ips typographus L. (Col., Scolytidae). J. Appl. Entomol. 1996, 120, 199–204. [Google Scholar] [CrossRef]
- Grucmanová, S.; Holuša, J.; Nermut’, J. Nematodes associated with the double-spined bark beetle Ips duplicatus (Coleoptera: Curculionidae) in central Europe. J. Appl. Entomol. 2014, 138, 723–732. [Google Scholar] [CrossRef]
- Tenkáčová, I.; Mituch, J. A contribution to the knowledge of nematofauna of Scolytidae bark beetles in norway spruce in forest park in Kosice. Lesn. Cas. For. J. 1986, 32, 381–387. [Google Scholar]
- Tenkáčová, I.; Mituch, J. Nematodes new for the fauna of the Czechoslovak Socialist Republic with the affinity to Scolytids (Coleoptera: Scolytidae). Helminthologia 1987, 24, 281–291. [Google Scholar]
- Tenkáčová, I.; Mituch, J. Nematodes of bark beetles (Coleoptera: Scolytidae) from Tatra National Park. Zbornık Prac o Tatranskom Narodnom Parku 1991, 31, 173–182. [Google Scholar]
- Schroeder, L.M. Escape in space from enemies: A comparison between stands with and without enhanced densities of the spruce bark beetle. Agric. For. Entomol. 2007, 9, 85–91. [Google Scholar] [CrossRef]

| Study Sites | Country | GPS Coordinates | Year of Collection | Pheromone Lure | Altitude (m a.s.l.) | |
|---|---|---|---|---|---|---|
| N | E | |||||
| Nås | SWE | 60.4677 | 14.5003 | 2014 | ID Ecolure | 232 |
| Siljansfors | SWE | 60.9730 | 15.0578 | 2014 | ID Ecolure | 324 |
| Vansbro | SWE | 60.5229 | 14.2389 | 2014 | ID Ecolure | 229 |
| Vindeln | SWE | 64.2000 | 19.7833 | 2014 | ID Ecolure | 291 |
| Petkówka | PL | 49.7333 | 19.2333 | 2015; 2016 | Duplodor | 668 |
| Rajcza | PL | 49.7666 | 19.2333 | 2015; 2016 | Duplodor | 646 |
| Romanka Górna I | PL | 49.5805 | 19.2246 | 2016 | Duplodor | 829 |
| Romanka Górna II | PL | 49.9338 | 19.3989 | 2015 | ID Ecolure | 1009 |
| Sopotnia Dolna | PL | 49.9350 | 19.4664 | 2015 | ID Ecolure | 953 |
| Tokarnia | PL | 49.9833 | 19.9833 | 2015 | ID Ecolure | 688 |
| Ujsoły | PL | 49.7508 | 19.2009 | 2015; 2016 | Duplodor | 859 |
| Złatna | PL | 49.4833 | 19.1666 | 2015 | ID Ecolure | 638 |
| Hlubočky | CZ | 49.6920 | 17.4146 | 2013 | ID Ecolure | 382 |
| Jílové u Prahy I | CZ | 49.8866 | 14.5055 | 2016 | Pheagr IDU | 354 |
| Jílové u Prahy II | CZ | 49.9166 | 14.5071 | 2016 | Pheagr IDU | 457 |
| Pustá Polom | CZ | 49.8510 | 18.0242 | 2014 | ID Ecolure | 454 |
| Calafindești | RO | 47.8513 | 26.1459 | 2011 | exp. lure | 497 |
| Ionu | RO | 47.6134 | 25.4817 | 2013 | exp. lure | 1080 |
| Solca | RO | 47.7000 | 25.7963 | 2013 | exp. lure | 625 |
| Sucevița | RO | 47.7767 | 25.4817 | 2013 | exp. lure | 605 |
| Todirești | RO | 47.7127 | 26.0328 | 2013 | exp. lure | 415 |
| Study Sites | Country | N | L.d. (%) | C.t. (%) | I.n. (%) | H.n. (%) |
|---|---|---|---|---|---|---|
| Nås | SWE | 46 | 39.1 | - | 15.2 | - |
| Siljansfors | SWE | 70 | 21.4 | 1.43 | 10.0 | 4.3 |
| Vansbro | SWE | 156 | 16.7 | - | 3.2 | 1.3 |
| Vindeln | SWE | 72 | 23.6 | - | 11.1 | 5.6 |
| Petkówka | PL | 107 | 19.6 | - | 3.8 | 4.6 |
| Rajcza | PL | 103 | 13.6 | - | 14.1 | 5.5 |
| Romanka Górna I | PL | 27 | 7.4 | - | 14.8 | - |
| Romanka Górna II | PL | 192 | 20.8 | - | 4.7 | 7.3 |
| Sopotnia Dolna | PL | 35 | 25.7 | - | 5.7 | 2.9 |
| Tokarnia | PL | 139 | 19.4 | - | 6.5 | 3.6 |
| Ujsoły | PL | 22 | 9.1 | - | 13.6 | 9.1 |
| Złatna | PL | 20 | 10.0 | - | 10.0 | - |
| Hlubočky | CZ | 22 | 13.6 | - | 18.2 | 4.6 |
| Jílové u Prahy I | CZ | 18 | - | - | 5.6 | - |
| Jílové u Prahy II | CZ | 43 | 7.0 | 2.3 | 4.7 | 4.7 |
| Pustá Polom | CZ | 237 | 27.4 | 0.8 | 10.1 | 1.7 |
| Calafindești | RO | 20 | 20.0 | - | 10.0 | - |
| Ionu | RO | 33 | 18.2 | - | 12.1 | 3.0 |
| Solca | RO | 80 | 11.3 | - | 3.8 | 3.8 |
| Sucevița | RO | 45 | 8.9 | - | 13.3 | 6.7 |
| Todirești | RO | 52 | 1.9 | - | 5.8 | 9.6 |
| Distance Class | Distance Centre | Moran’s I | p |
|---|---|---|---|
| 1 | 45.2 | 0.1 | 0.6 |
| 2 | 306.6 | 0.1 | 0.7 |
| 3 | 650.3 | −0.2 | 0.2 |
| 4 | 877.8 | −0.1 | 0.6 |
| 5 | 1137.8 | 0.1 | 0.7 |
| 6 | 1510.1 | −0.1 | 0.9 |
| 7 | 1975.8 | −0.4 | 0.1 |
| Variable | VIF | t Value a | p Value |
|---|---|---|---|
| Constant | 3.1 | 0.01 | |
| Latitude | 1.1 | 3.5 | 0.01 |
| C. typographi | 1.3 | 1.4 | 0.18 |
| Intestinal nematodes | 1.4 | 0.8 | 0.43 |
| Nematodes in hemolymph | 1.1 | −0.8 | 0.46 |
| altitude | 1.4 | 3.8 | 0.01 |
| number | 1.1 | 2.9 | 0.02 |
| year | 1.6 | −3.4 | 0.01 |
| Variable | VIF | t Value | p Value |
|---|---|---|---|
| Constant | 3.9 | 0.002 | |
| Latitude | 1.0 | 4.0 | 0.002 |
| Altitude | 1.2 | 3.7 | 0.002 |
| Number | 1.0 | 2.8 | 0.020 |
| Year | 1.1 | −3.9 | 0.002 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimová, S.; Resnerová, K.; Vanická, H.; Horák, J.; Trombik, J.; Kacprzyk, M.; Lindelöw, Å.; Duduman, M.-L.; Holuša, J. Infection Levels of the Microsporidium Larssoniella duplicati in Populations of the Invasive Bark Beetle Ips duplicatus: From Native to New Outbreak Areas. Forests 2019, 10, 131. https://doi.org/10.3390/f10020131
Zimová S, Resnerová K, Vanická H, Horák J, Trombik J, Kacprzyk M, Lindelöw Å, Duduman M-L, Holuša J. Infection Levels of the Microsporidium Larssoniella duplicati in Populations of the Invasive Bark Beetle Ips duplicatus: From Native to New Outbreak Areas. Forests. 2019; 10(2):131. https://doi.org/10.3390/f10020131
Chicago/Turabian StyleZimová, Soňa, Karolina Resnerová, Hana Vanická, Jakub Horák, Jiří Trombik, Magdalena Kacprzyk, Åke Lindelöw, Mihai-Leonard Duduman, and Jaroslav Holuša. 2019. "Infection Levels of the Microsporidium Larssoniella duplicati in Populations of the Invasive Bark Beetle Ips duplicatus: From Native to New Outbreak Areas" Forests 10, no. 2: 131. https://doi.org/10.3390/f10020131