Storage of carbon dioxide from a power station or industrial facilities can be feasibly done as climate change mitigation options in subsurface formation within or below the ocean or under the ground. Considering the large-scale capture and storage, it is crucial to highlights the toxicity of CO2. The concentration in the atmosphere is around 0.04% but, if it increases up to 10%, the CO2 acts as an asphyxiant while, if it rises until 20%, the inhalation leads to rapid death. Therefore, once stored, the site will need to be monitored for many years to verify that CO2 can never be released to the atmosphere.
5.1. CO2 Storage as Clathrate Hydrates
Subsurface CO
2 permanent sequestration through clathrate hydrates formation is a novel method to mitigate global warming. Gas hydrate are clathrate compounds in which some guest gas molecules (e.g., CO
2, CH
4, C
2H
6) are encapsulated in water molecules. The gas hydrate form under low temperature and high-pressure conditions via hydrogen bonding between water molecules. Reserves of methane in hydrate structure are abundant in geological accumulations in offshore and permafrost environments, and they exceed the all-carbon fossil fuel [
47].
Three methods are used to shift the equilibrium and produce methane from oceanic sediments: (i) thermal stimulation through direct heating or injection of heated fluid; (ii) depressurization is the preferred technique for driving gas hydrate dissociation; (iii) injection of chemical inhibitors; (iv) gas swapping [
48]. The thermal stimulation method requires a continuous energy source to raise the hydrate temperature above the stability point. The depressurization method decreases the hydrate pressure under the stability point, causing hydrate dissociation. Inhibitor injection involves the injection of a compound at isobaric conditions that shift the equilibrium point to the lower temperature. The injection of CO
2 molecule into NGH deposits involves the replacement of CH
4 into the water cages that leads to produce methane and store carbon dioxide.
CO
2 forms a stable hydrate structure at lower pressure conditions than CH
4 hydrates at the same temperature, as it is showed in
Figure 11. Therefore, CO
2 hydrates are more stable than CH
4 hydrates under certain conditions (i.e., CO
2 and CH
4 hydrates equilibrium curves intersect at around 10.5 °C and 75 bar), and it can displace the methane in the hydrate structure. In this way, it enables low carbon energy recovery (e.g., CH
4) while offsetting capture and transportation cost. Besides, CO
2 can re-occupy the pore space from methane recovery, maintaining the mechanical stability of the rock and preventing possible hazards of slope failures. Uchida et al. [
49] have demonstrated via experiment and theoretical calculation that, at a temperature below 10 °C, the equilibrium pressures of the CO
2 hydrates are lower than those of CH
4 hydrate.
CO
2 and CH
4 molecules form both the type SI of crystal-structure hydrate that consists of six medium cages and two small cages. During the hydrate formation, the CO
2 molecule, which is slightly larger than the CH
4 molecule, only occupies the medium cages, while the methane molecule occupies both medium and small cages. As a result, more CH
4 can form in the early stages. The heat of CO
2 hydrate formation (−57.9 kJ/mol) is greater than the heat of dissociation of CH
4 hydrates (54.5 kJ/mol). Therefore, a replacement reaction occurs in the later stages due to the favorable exothermic process [
51]. The exothermic nature of the CO
2 hydrates formation provides the heat of methane dissociation and increases the storage temperature. Therefore, the storage’s temperature is a critical parameter that has to remain below 10.5 °C to avoid CO
2 dissociation and CH
4 hydrate re-formation.
Lee et al. have examined CH4 and CO
2 molecule distribution over the two cage sites (i.e., S and M) by Magic Angle Spinning Carbon-13 Nuclear Magnetic Resonance (MAS
13C NMR) into a hydrated sample. This analysis shows a limit to the CO
2 replacement degree. It was estimated that 64% of methane can be recoverable from the NGH reservoir, resulting, after only via the exchange of CO
2, a product hydrate with a CO
2/CH
4 ratio of 1.8 or greater at equilibrium condition. Otherwise, a kinetic test showed that the replacement reaction is almost complete in less than five hours, and the product hydrate results with a CO
2/CH
4 ratio of 1. Consequently, 50% of methane included in the reservoir can be replaced by CO
2, and therefore a complete methane recovery is not possible [
52].
The mixture of carbon dioxide and methane gas is present in the hydrate reservoir when the CO
2 is injected into the NGH. Adisasmito et al. proposed the following polynomial equation fitted to the experimental data to measure the gas mixture’s equilibrium condition in hydrate form [
53].
The equilibrium pressure
is measured in MPa, the temperature
is in °K, and
y is the mole percent of carbon dioxide in the vapor phase. The above equation was fitted for a temperature between 273 K and 288 K, while the maximum pressure of the CO
2 was selected not to exceed 4.37 MPa to avoid the formation of liquid CO
2. Indeed, at a pressure higher than 4.37 MPa, the dissociation of methane hydrate occurs at a higher temperature than the hydrate formation of liquid CO
2. Therefore, at high pressure, the CO
2 might not sequester as hydrate and can cause instability of the seafloor. The equilibrium condition of the hydrate mixtures is shown in
Figure 12.
The upper and lower limit of the phase equilibrium diagram represents the cases of pure methane and pure carbon dioxide hydrates, respectively. The equilibrium conditions for the mixed gases lie between these two limits. The figure shows that at a fixed temperature, the equilibrium conditions move to lower pressure and, at a fixed pressure, they move to a higher temperature when the CO2 mole fraction in the mixture increases.
The experiments above are conducted in the bulk phase; however, the dissociation of methane hydrate and its replacement via carbon dioxide occur in sediment reservoirs. Therefore, this process needs to be studied in porous media.
Figure 13 compares the hydrates equilibrium conditions in porous media with those in the bulk phase [
54].
The experimental data are measured mainly in porous silica gel and porous glass, and they are represented via symbols, while the lines represent value obtained by correlations. The figure shows that the equilibrium pressure is higher at a temperature while, at a pressure, the equilibrium temperature is lower in porous media than in the bulk phase. Besides, from the last two figures, we can deduce that increasing the CO
2 mole fraction in the hydrate deposits will reduce the favorable range of temperature and pressure. Consequently, an accurate range selection is needed for simultaneous methane production and carbon dioxide sequestration [
54].
The processes of hydrate formation and dissociation in porous media are different to respect those in the bulk phase. In porous media, the hydrate will first form in the smaller size pores as the temperature decrease. On the other hand, if the temperature increases, the hydrates will first dissociate in the largest pore. The injected CO
2 can form hydrate into a pore throat blocking the pore. As a result, the hydrate particles can isolate large quantities of methane hydrate in a deeper pore and hampers further methane dissociation and carbon dioxide sequestration. Clennest et al. have predicted the hysteresis cycle between crystallization temperature and the dissociation of the clathrates in porous media [
55]. Concerning the heat of dissociation and formation of the hydrates, several researchers have yielded conflicting results and therefore need further investigation [
54].
Gambelli et al. have tested the CO
2 replacement into NGH deposit via a combination of two strategies. Once the CH
4 hydrate formation is carried out, the replacement phase is performed via depressurization and CO
2 injection. The quantity of CO
2 stored depends on the sum of two contributions: the methane replacement and an ex-novo CO
2 hydrate formation. The CO
2-CH
4 exchange in methane hydrates is favorable; however, the new CO
2 hydrate formation around and the superficial replacement process limit the transition of the CO
2 into the deeper layer inside the deposits and, therefore, the overall replacement process. As a result, the storage efficiency (i.e., the ratio between the moles of CO
2 permanently stored into the NGH and the total amount of CO
2 injected inside the deposit) is limited at 36% with a value of sand pore saturation degree equal to 7.6% [
56].
An experimental apparatus able to promote methane production via a combined CO
2 replacement and depressurization was tested by Zhao et al. [
57]. In this manuscript, they run two tests conducted with and without depressurization combined replacement method. This experiment shows the great benefit of the combined method, increasing methane replacement from 7% without depressurization to 25% with a mixed method [
57].
Japan and China have carried out methane production testing in oceanic NGH reservoir, showing the possibility and feasibility of methane production by depressurization. Four tests are conducted, three in Nankai Trough (Japan), and one in Shenhu Area, China. The first Japanese test was done in March 2013, but the methane production was interrupted only after six days due to the abrupt sand production. The other two tests were performed in the same location in 2017 but also, in this case, the trials lasted 12 and 24 days, respectively. China has extracted from an oceanic NGH reservoir via a single vertical well by depressurisation method in the same period. China’s test was performed in the South China Sea for 60 days. The average daily gas production rate falls between 2.9 × 10
3–8.3 × 10
3 in both test sites, with a peak of 2 × 10
4 m
3/day [
58].
5.2. Carbon Dioxide Replacement Cost and Conventional Storage Cost
The recovery of methane from NGH and its replacement with CO
2 molecule remains very challenging, and no previous work on the open literature have reported its cost. Only a few studies have commented on the economics of methane production from NGH reservoirs, and one of these is used to extrapolate the infrastructure’s data cost. In fact, Vedachalam et al. [
59] have analysed the techno-economic methane production based on the depressurization method. This method involves a vertical well that connects the NGH formation with a Floating Production Unit (FPU) with a pump able to depressurize the reservoir. The main cost parameters are summarized in the
Table 6 below:
We have assumed that the cost data summarized in
Table 6 for methane production via depressurization method are the same when a combined method (i.e., CO
2 replacement + depressurization) is applied. The main financial and technical parameters used as input in this simulation are summarized in
Table 7. The lifetime of the plant is set up at 15 years [
58]. We have assumed that the CO
2 only replaces methane hydrate and does not form a new hydrate to facilitate the economic simulation.
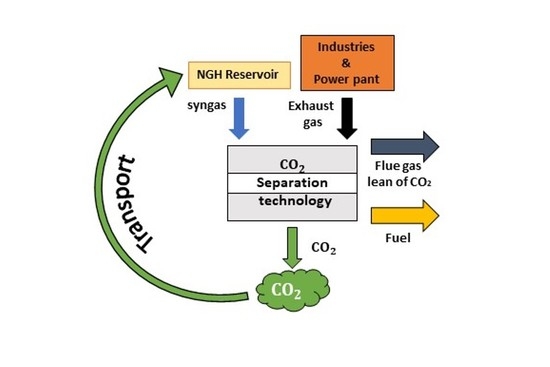
The tests defined the annual methane production performed both in Japan and China sites, and it was assumed that could be the same when the combined method is applied. The syngas produced from NGH is not composed only of methane but also the injected CO
2 that does not participate in the reaction [
56]. As a result, the produced syngas comprises 36% CH
4-66% CO
2 at the chosen storage efficiency. The economic analysis was conducted for different numbers of wells, and the minimum cost to store one ton of CO
2 is illustrated in
Figure 14.
Increasing the number of wells, the revenue derived from methane’s sale goes up because the methane production increases. Simultaneously, the amount of carbon dioxide that replaces the methane and is sequestrated as hydrate increases. As a result, the CO2 storage cost drops from 11.6 $/t to 5.8 $/t when the wells’ numbers pass from one to two, respectively. The minimum CO2 storage cost was obtained with ten wells, but there are no manuscripts that report many such wells working simultaneously to produce methane in the NGH reservoir. Therefore, the CO2 storage cost in NGH with two wells might be the representative value to compare this solution with the other possible storage sites.
Costs for geological storage are widely variable due to different reservoir types (e.g., onshore, offshore, depleted field, deep saline formation) and reservoir geology (e.g., depth, permeability). Therefore, storage cost is reported as a range, and the data are provided by different literature.
5.3. Methane Upgrade with Membrane-Based Separation Technology
Concerning the CO2 separation process, membrane technology is used to upgrade biogas, increasing methane content on NG/SNG, reaching the purity fixed by a gas network.
Natural gas is considered a helpful bridge fuel to decrease greenhouse gas emissions. Purification and quality upgrade are necessary to use several natural gas reservoirs at low methane concentration as well as several processes are enough to carbon conversion and SNG production. For this purpose, membrane technology is applied to separate CH4 from a mixture of gases composed by CO2, CH4, N2, H2O, and heavy hydrocarbons.
Commonly used glassy polymers for gas separation are polyimides (PI), polysulfone (PSF), polycarbonates (PC), while the mainly used rubber membranes are polyurethane (PU) and polydimethylsiloxane (PDMS). Among the PI polymers, Matrimid membranes are the most interesting; it is inflexible, strong and presents a CO
2 permeability of 12.7 barrer and CO
2/CH
4 ideal selectivity near to 40, at 20 bar [
61].
PSF membranes are produced in three ways (asymmetric, dense, and composite), and they have CO
2 and CH
4 permeability of 12.33 and 4.69, respectively, while the CO
2/CH
4 selectivity is equal to 3.37 [
62]. Carbon dioxide sequestration is relevant nowadays, and PDMS membranes possess excellent CO
2 permeability, high thermal stability, and a low rate of aging.
Also, the mixed matrix membrane is used to upgrade natural gas. Graphene oxide (GO) has been incorporated into the polyethylene oxide matrix (PEO) for sustainable CO
2 capture obtaining mechanical properties improvement. Permeability is enhanced by taking advantage of increased fractional free volume. As the GO contents increase from 0 to 1.0%wt, the H
2, N
2, and CO
2 (10 atm) permeabilities increase from 27.3, 5.84, and 280 barrer to 47.7, 8.5 and 474 barrer, respectively [
63].
Simultaneous improvement in CO
2 permeability and CO
2/CH
4 selectivity can result in dispersing NOTT-300, a new MOF, in the Pebax1657 matrix. Increasing the content of BOF CO
2 permeability reached 395 barrer, while CO
2/N
2 and CO
2/CH
4 selectivity reached 61 and 36, respectively, at 10 bar [
64].
Several authors have analysed the techno-economic feasibility of CO
2 removal from natural gas via membrane technology, more of them with a CO
2 content between 10% and 50% into the syngas. Yunhan Chu et al. have performed an HYSYS simulation and a cost evaluation. Assuming that 100
$/m
2 is the carbon-based membrane cost, the use of two-stage with recycling and a feed syngas with 50% of CO
2, the natural gas processing cost results in 0.044
$/Nm
3 of natural gas achieving 98% of CH
4 in the retentate and 2% of losses [
65].
Gilassi et al. have performed a new optimization model to determine the optimum module number while minimizing the cost. The model has revealed that the use of two membrane units is enough to rich a pure output stream composed up to 98% of methane and reduce the methane losses above one percent. The biogas separation cost increases almost linearly with the content of CO
2 into the feed syngas. The biogas separation cost varies from 0.045
$/Nm
3 to 0.09
$/Nm
3 of CH
4 with 10% and 40% of CO
2 contents in the feed syngas, respectively [
66].
The syngas produced during the methane extraction and simultaneous carbon dioxide sequestration from the NGH reservoir might contain 64% of CO
2. This value is higher than that estimated by the authors previously cited. Nevertheless, a two-stage membrane separation process to upgrade the methane content until 98% can also be used for this application, as shown in
Figure 15. As a result, the separation cost can be equal to 0.126
$/Nm
3 with 64% of CO
2 from its linearization. Therefore, the CO
2 separation cost is 0.036
$/ton of CO
2 as evaluated in Equation (10).
is the cost to separate the CO2 in $/ton of CO2, is the biogas separation cost, and are the mole fractions of methane and carbon dioxide in the syngas, respectively, and is the density of carbon dioxide equal to 1.976 ton/Nm3 of CO2.