Manufacturing differences introduce variations in the structures and chemical compositions of cells. Moreover, its exact composition is a manufacturer’s secret and the set of material properties of a cell are usually inaccessible for a particular case. Consequently, little detailed preliminary information about the electrode geometries, porosities, particle sizes, composition of the electrolyte and all associated parameters such as specific surface areas, active electrode surface, electrode and cell thicknesses, ionic conductivity, diffusion coefficients was available. As was mentioned earlier, the complexity of the multi-particle battery model will be beyond the expected target of application due to the numerous unknowns and unreasonably profound resolution. Instead, an attempt was made to apply a simplified model for parameter identification that should offer a reasonable compromise between resolution and computability. Therefore, a simple characteristic cell was established by adopting some of the necessary electrochemical parameters from the following literature that could not be indirectly measured.
3.1. Characteristic Cell Model
The cell consists of a porous double-sided NMC cathode and a double-sided graphite anode. Most of the material parameters were taken from Carelli et al.’s work [
53] since they investigated the same type of NMC and carbon-based cell as us. According to this study, the radii of the cathode
and anode
particles were 5
and 10
, respectively. These values are also in agreement with the manufacturer’s datasheet of NMC111 [
54]. The initial volume fraction of the solid particles in the cathode
is 0.5224, while its void (electrolyte) fraction
is 0.2976. The gas phase is neglected in this case. The volume fraction of solid particles in the anode
is 0.5073 and its void fraction
is 0.4526. The recommended values for the porosity of the separator (in the liquid phase)
are within the range of 0.4–0.6 and for the thickness of the separator
are between 20–25
[
55]. According to Newman’s work [
56], the porosity of the separator was set at 0.4.
was arbitrarily chosen to be 22.5
as an average value within the recommended range. The Bruggeman coefficient
was chosen to be 1.5 for both of the electrodes based on a specific study on Bruggeman coefficients in NMC electrodes [
35]. The initial Li concentration in the LiN
0.3M
0.3C
0.3O
2 cathode
was 49.000 mole/m
3, while the initial Li concentration in the MCMB graphite anode
was 31.507 mole/m
3. These are material constraints and depend on the compositions of the electrodes. A commonly used electrolyte is LiPF
6 in an organic solvent. Even though some of the properties of the electrolyte (e.g., ionic conductivity, temperature range) depend on the mixture and volume ratios of alkyl carbonate solvents such as ethylene carbonate (EC), dimethyl carbonate (DMC), diethyl carbonate (DEC) and ethyl methyl carbonate (EMC) [
57], the initial salt concentration
of 1 mole/dm
3 and transference number
of 0.363 are similar in these electrolytes according to Valoen et al. [
58]. Most of the electrolytes in recent cells are based on the salt LiPF
6 in some of these alkyl carbonate mixtures with a salt concentration of 1 mole/dm
3. However, the exact chemical formulation of the electrolyte is unknown. In Serena et al.’s work [
53], it is shown that Nyman et al., Zhang et al. and Ecker et al. gathered values of the ionic conductivity
of different electrolyte mixtures. Based on their studies,
was assumed to have an average value of 0.87 S/m. A schematic diagram of the cell structure can be seen in
Figure 2.
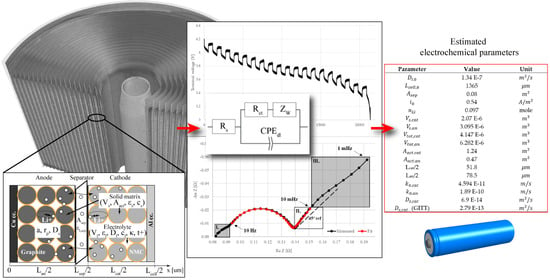
3.2. Extended Randles Equivalent Circuit Model
As was mentioned at the end of the Introduction, it is our aim to determine the core electrochemical parameters concerning liquid-phase diffusion impedance. These parameters were determined by fitting an extended Randles equivalent circuit on EIS data where the non-idealities of diffusion and migration were implemented by distributed elements. The extended Randles circuit can be seen in
Figure 3.
Rs and
Rct stand for the serial and charge-transfer resistances of the cell, respectively. The classically used double-layer capacitor in Randles circuits was now replaced by a CPE to implement a more realistic, “leaky” capacitor. On the other hand, the standard Warburg element used for ideal diffusion modeling in the Randles circuits was substituted by its generalized alternative to handle non-idealities. Batteries with porous electrodes such as ours are expected to exhibit non-Fickian behavior which can be observed in their phase angles other than 45°. Several authors have extensively investigated the source of these non-idealities and have pointed to multi-phase and multi-scale diffusion phenomena in porous electrodes [
59,
60], to diffusion coupled with migration [
61,
62], and/or to diffusion in non-conventional space [
39,
63,
64] as root causes of the non-Fickian behavior.
It is also important to note that there are finite- and infinite-length Warburg elements and CPEs in the tool set which at first sight seem to also be useful alternatives to non-ideal diffusion modeling. However, finite-length Warburg elements are dedicated to model transmissive or reflective diffusion behavior at the very low frequencies through their hyperbolic functions. Since measuring impedance at very low frequencies is unaffordable, there is usually insufficient data from that region which makes the fitting of finite-length Warburg elements unreasonable. Although a standard infinite-length Warburg element does not suffer from this problem, it is inaccurate for non-ideal diffusion modeling.
Wang [
65] and Franceschetti together with MacDonald [
61] have laid down the fundamentals of the theory and mathematical background concerning generalized Warburg impedance. According to these papers, the generalized form of the Warburg element can be written in the following form:
where 0 <
n < 1,
A denotes a Warburg constant and
. Wang notes in his paper [
65] that a circuit element with an impedance described by (1) is sometimes referred to as a CPE. In terms of a core parameter estimation process, liquid-phase diffusion modeling is of most interest. Therefore, only the relationships used for liquid-phase diffusion modeling were focused on in the following.
First, Equation (1) was modified by applying the Euler’s formula and our nomenclature in order to make the mathematical deductions easier to follow. By implementing some simple mathematical operations, Equation (1) can be rewritten in the form of
. By applying Euler’s formula, the following equation can be derived, which is in accordance with the form presented by the authors on page 12 [
66]:
where
denotes the Warburg coefficient and
is a so-called dispersion parameter. Following the analogy of classical Warburg relationships, which is detailed for example on page 380 of A. Bard et al.’s book [
67],
is the fraction of the Warburg resistance
and
as follows:
where
denotes the limiting diffusion resistance as a function of mobility [
32].
stands for the fraction of the effective characteristic length
and the related effective diffusion coefficient
:
where
is the characteristic length and
is the related effective diffusion coefficient.
Expressing the generalized Warburg impedance in an electrochemical form is useful to determine the material parameters. Based on the electrochemical form of semi-infinite planar diffusion impedance
(Equation (88) of [
47]) when “inter-electrode distance is much larger than the characteristic diffusion length” and a supporting electrolyte may have been used, (2) can be rewritten in the following form:
where
R denotes the universal gas constant,
T stands for the absolute temperature,
z represents the amount of charge involved in the reaction,
F refers to Faraday‘s constant,
is the Li-ion concentration in the diffusion material,
denotes the intrin9sic Li-ion diffusion coefficient, and
stands for the cross-sectional area of the cell perpendicular to the direction of ion transport. In this case,
is equal to the cross-sectional area of the separator. 0 written in subscript denotes its intrinsic property instead of effective value. Before moving forward, it should be clarified that our technique seems to have a supporting electrolyte as most of the state-of-the-art Li-ion batteries do in order to minimalize their ionic resistances and improve the chemical stability of their electrolytes. Therefore, the diffusion of PF
6 in the electrolyte is neglected. This concept has been confirmed by researchers who pointed out that the diffusion of Li
+ controls the rate of ion transport within the range of salt concentrations of 0–2.5 M [
68].
It is also important to note that (5) implies that the following criteria should be kept: excitation is quasi-equilibrium, that is,
as well as
and either the oxidant or reductant is not in the solution. By taking the real part of the Warburg impedance from Equation (5), the following equation can be derived:
where
denotes the real part of the Warburg coefficient. Equation (6) serves as a basis for estimating electrochemical parameters in the following. There are four unknown core variables, namely
,
,
and
, and four independent equations are required to find the solution. Wang et al. [
34] presented an approach to determine
by fitting the real part of the impedance data against
but is only valid for Fickian diffusion. Nevertheless, this method has been extended to the generalized case
that can be mathematically expressed as:
where R’ denotes an offset and
represents the gradient of the trend. Since macroscale electrochemical parameters are sought, the characteristic diffusion length expressed by Equation (4) was scaled to the inter-electrode level to obtain:
where
denotes the intrinsic thickness of the unity cell,
represents the Bruggeman coefficient and
stands for the liquid fraction in the separator. In Equation (8),
can be experimentally derived from the instantaneous resistance of the cell. The instantaneous resistance of the cell refers to
shown in
Figure 3 which can be quantified either from EIS or simple time-domain measurements. Measuring
from EIS is trivial. In the case of time-domain measurements, J. Lindberg et al. [
69] derived a mathematical formula to calculate
according to:
where
denotes the drop in the terminal voltage caused by steady-state concentration gradients for a given cell thickness and
stands for the effective ionic conductivity. The first term on the right-hand side of Equation (9) is
while the second term is the diffusion resistance. Since
obtained from the instantaneous voltage drop is of interest, the second term was neglected. By integrating the first term in Equation (9) into the overall cell thickness, the following form was derived:
The coefficient of the term on the left-hand side can be expressed by the cell resistance which results in a simplified voltage form:
where
denotes the initial terminal voltage of the cell. By measuring the terminal voltages of the cell against different load currents,
can be determined by the gradient of the trend. By rearranging (10) in the form
:
In (12),
,
, and
can be deduced from the characteristic cell, while the values of
,
,
and
can be measured. Consequently, if Equations (8) and (12) are combined and inserted into (6),
can be expressed as:
Once has been determined, and can also be estimated by Equations (12) and (8), respectively.
Up to this point, all the relationships which allow diffusion-related core parameters to be obtained have been presented. In the following, how our technique is able to determine the exchange current density
and reaction rate constant
by calculating characteristic geometrical parameters is shown. The word “characteristic” is used to indicate that the values are taken from the dataset of the characteristic cell so were not measured in this work. Here, it is highlighted that the parameters being estimated in the following might be different for the anode and cathode, moreover, their individual values could be measured by three-electrode measurements with Li metal as a reference. Although this method offers a solution for the lumped-parameter problem, three-electrode measurements still introduce issues concerning the placement of a reference electrode. Furthermore, our technique targets realistic in situ measurements of commercial instead of special experimental batteries where individual characteristics are inaccessible without cell abuse. This led us to consider
as a lumped parameter which represents an average rate of both electrodes simultaneously.
can be calculated by the simplified Butler-Volmer equation presented on page 99 in the book by Bard and Faulkner [
67] that is valid for low overpotentials:
where
Rct denotes the charge transfer resistance that can be experimentally determined by fitting on EIS data within the mid-frequency range.
In order to obtain
, first some characteristic geometrical parameters must be calculated. The following calculations are based on our assumption that the total utilizable amount of charge the electrodes can accumulate is equal to the total number of Li ions in the cell which is given by the manufacturer. Therefore, it is necessary to calculate the utilizable amount of Li
first by rearranging Faraday’s equation in the form of
according to:
where
Q denotes the nominal capacity of the cell. After that, the volume of the solid
was calculated in the given electrode by the ratio of the utilizable amount of active particles per volume to the available number of active particles. Mathematically, this is:
where
denotes the concentration of Li ions in the given electrode and the utilizable amount of Li ions in the solid phases
reflects the material constraints the electrodes naturally have. According to the definition of porosity, it is possible to estimate the total volume of the given electrode
based on the solid and liquid fractions:
Another important geometrical parameter is the active surface area
which yields the area of the solid electrode particles surrounded by the electrolyte. It is the product of the specific particle surface and the total volume of the given electrode and can be expressed by:
The specific surface area of the particles of the electrode is
. The thickness of the given electrode can be derived by dividing Equation (17) by Equation (18):
,
,
and
can be calculated for both electrodes to roughly approximate their internal geometrical dimensions. Obviously, the estimated values of parameters expressed in (16)–(19) can moderately deviate from real data since they are based on both the assumptions of the characteristic cell and nominal values from manufacturer datasheets. Nevertheless, knowing these internal parameters—even with a moderate degree of uncertainty—is beneficial with regard to geometrical scaling of cells. It should be highlighted here that the most accurate geometrical data is accessible by using any of the direct measurement techniques mentioned in the Introduction. However, those procedures are unavailable for in situ measurements without having to dismantle the cell.
can be calculated for both of the electrodes in a quasi-equilibrium state when the overpotential is small (<15 mV) by the fraction of
for the given electrode
and the concentration of Li ions at the electrode surface
. Mathematically, this can be derived from the Butler-Volmer equation for a limiting case when the overpotential is very small. The formula from page 210 of Newman’s book [
70] was adopted, that is:
where the units are
,
,
and
.
is assumed to be equal to the solid concentration of the electrode
. Since
was measured as an average value of exchange current rates of the electrodes,
also becomes a lumped parameter. Considering the parameters detailed so far, the reaction rate
and
implement the main terminal voltage characteristics during long-term battery usage in the finite element model. Even though these parameters usually change with the solid- and liquid-phase concentrations, the initial values of these parameters play an important role in modeling true battery dynamics.
An attempt was also made to estimate the order of magnitude of
Ds without any additional measurements by assuming that the calculation of
can be treated by considering the particle radius
rp as a characteristic diffusion length. In other words, this idea implies an average approximation of the entire diffusion impedance curve by the tail part. In this sense,
Ds was approximated by the formula taken from Krewer et al.’s paper [
13] which gives the relationship between the diffusion time-constant, particle size and solid-phase diffusion coefficient according to:
where
denotes an average particle radius of the given electrode.
In order to obtain a more reliable value of
, GITT measurements were taken. The main procedure is detailed in the Materials and Methods section.
was determined by the following relationship adapted from the literature [
71]:
where
denotes the duration of a current pulse,
represents the number of moles of Li ions,
stands for the molar volume of the cathode,
refers to the change in voltage during the relaxation phase and
is the change in voltage during the discharge period. Choi et al. [
72] pointed out that calculation of
by GITT is only possible for single-phase reaction which cathode shows.