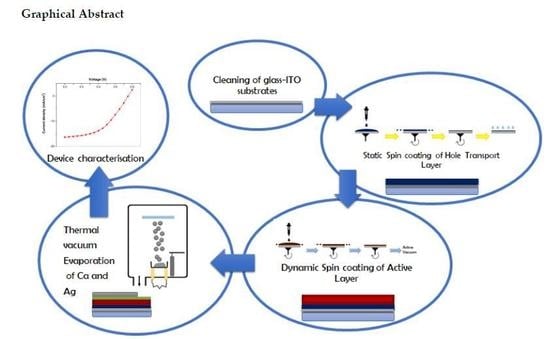
Building an Organic Solar Cell: Fundamental Procedures for Device Fabrication
Abstract
:1. Introduction
2. Pedagogical Goals
- Learning of the step-by-step method of construction for an efficient bulk heterojunction organic solar cell device.
- Familiarizing with the fabrication and characterization techniques and equipment used.
- Understanding of the importance of optimizing device performance through enhancing the optical, electrical, and morphological properties of the materials selected and perfecting the experimental parameters.
- Introducing students to the operation principle of a bulk heterojunction organic solar cell.
3. Principles of Operation and Device Structure
- (a)
- Sunlight is absorbed in the form of photons by the photoactive layer which consists of an electron donor material (PTB7) and an electron acceptor material (PC71BM) which are intimately mixed together.
- (b)
- When a photon is absorbed by the donor material, an electron is excited, leaving behind a positively hole. The electron and hole are bound by Coulombic forces forming a quasiparticle known as an exciton.
- (c)
- The exciton diffuses toward the donor–acceptor interface where it is dissociated. Due to the intimate mixing of the donor and acceptor materials, the interface where excitons dissociate, and free carriers are generated, is extended and therefore optimized.
- (d)
- The free electron travels through the donor and hole transport layer and is eventually collected at the anode, while the free hole travels through the acceptor material and electron transport layer, eventually being collected at the cathode. The flow and collection of these free carriers is essentially how electrical current is generated with photons from sunlight as the source.
4. Experimental Section
4.1. Safety Precautions
4.2. Materials and Equipment
4.3. Experimental Procedure—Logistics
4.4. First Day—Session 1
4.4.1. Four Step Cleaning Process
4.4.2. Preparation of the Bulk Heterojunction Active Layer Blend
4.5. Second Day—Session 2
4.5.1. Hole Transport Layer
4.5.2. Deposition of the Hole Transport Layer
4.5.3. Transmittance Measurement of PEDOT:PSS
4.5.4. Morphology Characterization of PEDOT:PSS—Atomic Force Microscopy
4.5.5. Sheet Resistance Measurement of PEDOT:PSS
4.5.6. Deposition of PTB7:PC71BM via Dynamic Spin Coating Method
4.5.7. Absorption Measurement of PTB7:PC71BM via UV-Vis Spectroscopy
4.5.8. Morphology Characterization of PTB7:PC71BM—Atomic Force Microscopy
4.5.9. Sheet Resistance Measurement of PTB7:PC71BM
4.5.10. Deposition of the Ca—ETL and the Ag cathode
4.5.11. Carrier Mobility: Hole Mobility—Electron Mobility
5. Device Characterization
5.1. Third Day—Session 3
5.1.1. Electrical Performance—Organic Solar Cell Device Evaluation—Photovoltaic Performance
5.1.2. External Quantum Efficiency
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mickey, C.D. Solar photovoltaic cells. J. Chem. Educ. 1981, 58, 418. [Google Scholar] [CrossRef]
- Abruña, H.D. Energy in the Age of Sustainability. J. Chem. Educ. 2013, 90, 1411–1413. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Duan, C.; Li, M.; Hu, Z.; Wang, J.; Liu, F.; Li, N.; Brabec, C.J.; Janssen, R.A.J.; et al. Morphology Optimization via Side Chain Engineering Enables All-Polymer Solar Cells with Excellent Fill Factor and Stability. J. Am. Chem. Soc. 2018, 140, 8934–8943. [Google Scholar] [CrossRef] [Green Version]
- Smestad, G.P.; Grätzel, M. Demonstrating Electron Transfer and Nanotechnology: A Natural Dye-Sensitized Nanocrystalline Energy Converter. J. Chem. Educ. 1998, 75, 752. [Google Scholar] [CrossRef]
- Dubey, A.; Adhikari, N.; Mabrouk, S.; Wu, F.; Chen, K.; Yang, S.; Qiao, Q. Strategic Review on Processing Routes towards Highly Efficient Perovskite Solar Cells. J. Mater. Chem. A 2018, 6, 2406–2431. [Google Scholar] [CrossRef]
- Li, F.; Liu, M. Recent efficient strategies for improving the moisture stability of perovskite solar cells. J. Mater. Chem. A 2017, 5, 15447–15459. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, M.A.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef]
- Cherrette, V.L.; Hutcherson, C.J.; Barnett, J.L.; So, M.C. Fabrication and Characterization of Perovskite Solar Cells: An Integrated Laboratory Experience. J. Chem. Educ. 2018, 95, 631–635. [Google Scholar] [CrossRef]
- Renny, A.; Yang, C.; Anthony, R.; Lunt, R.R. Luminescent Solar Concentrator Paintings: Connecting Art and Energy. J. Chem. Educ. 2018, 95, 1161–1166. [Google Scholar] [CrossRef]
- Lai, T.H.; Tsang, S.W.; Manders, J.R.; Chen, S.; So, F. Properties of interlayer for organic photovoltaics. Mater. Today 2013, 16, 424–432. [Google Scholar] [CrossRef]
- Leo, K. Organic photovoltaics. Nat. Rev. Mater. 2016, 1, 16056. [Google Scholar] [CrossRef]
- Marinova, N.; Valero, S.; Delgado, J.L. Organic and perovskite solar cells: Working principles, materials and interfaces. J. Colloid Interface Sci. 2017, 488, 373–389. [Google Scholar] [CrossRef]
- Lattante, S. Electron and hole transport layers: Their use in inverted bulk heterojunction polymer solar cells. Electronics 2014, 3, 132–164. [Google Scholar] [CrossRef]
- Seo, J.H.; Koo, J.R.; Lee, S.J.; Seo, B.M.; Kim, Y.K. Efficient Organic Photovoltaic Devices by Using PEDOT:PSS with Excellent Hole Extraction Ability. J. Nanosci. Nanotechnol. 2011, 11, 7307–7310. [Google Scholar] [CrossRef]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photonics 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Cheng, P.; Liu, Y.; Chang, S.-Y.; Li, T.; Sun, P.; Wang, R.; Cheng, H.-W.; Huang, T.; Meng, L.; Nuryyeva, S.; et al. Efficient Tandem Organic Photovoltaics with Tunable Rear Sub-cells. Joule 2019, 3, 432–442. [Google Scholar] [CrossRef]
- Stylianakis, M.M.; Konios, D.; Viskadouros, G.; Vernardou, D.; Katsarakis, N.; Koudoumas, E.; Anastasiadis, S.H.; Stratakis, E.; Kymakis, E. Ternary organic solar cells incorporating zinc phthalocyanine with improved performance exceeding 8.5%. Dyes Pigments 2017, 146, 408–413. [Google Scholar] [CrossRef]
- Kakavelakis, G.; Del Rio Castillo, A.E.; Pellegrini, V.; Ansaldo, A.; Tzourmpakis, P.; Brescia, R.; Prato, M.; Stratakis, E.; Kymakis, E.; Bonaccorso, F. Size-Tuning of WSe2 Flakes for High Efficiency Inverted Organic Solar Cells. ACS Nano 2017, 11, 3517–3531. [Google Scholar] [CrossRef]
- Sigma-Aldrich—P3HT—Poly(3-hexylthiophene-2,5-diyl) Regioregular, Electronic Grade, 99.995% Trace Metals Basis, Average Mn 30,000–60,000. Available online: http://www.sigmaaldrich.com/ (accessed on 23 April 2019).
- He, Z.; Zhong, Z.; Su, S.; Xu, M.; Wu, H.; Yong, C. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photonics 2012, 6, 591–595. [Google Scholar] [CrossRef]
- Benten, H.; Mori, D.; Ohkita, H.; Ito, S. Recent research progress of polymer donor/polymer acceptor blend solar cells. J. Mater. Chem. A 2016, 4, 5340–5365. [Google Scholar] [CrossRef] [Green Version]
- To, C.H.; Ng, A.; Dong, Q.; Djurisič, A.B.; Zapien, J.A.; Chan, W.K.; Surya, C. Effect of PTB7 Properties on the Performance of PTB7:PC71BM Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 13198–13207. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Li, Y.; Xu, Z.; Zhao, S.; Zhao, L.; Zhao, J. Enhanced performance and morphological evolution of PTB7: PC71BM polymer solar cells by using solvent mixtures with different additives. Phys. Chem. Chem. Phys. 2015, 17, 8053–8060. [Google Scholar] [CrossRef] [PubMed]
- Heraeus—Conductive Polymers. Available online: https://www.heraeus.com/ (accessed on 23 April 2019).
- Kim, Y.H.; Sachse, C.; Machala, L.M.; May, C.; Müller-Meskamp, L.; Leo, K. Highly Conductive PEDOT: PSS Electrode with Optimized Solvent and Thermal Post-Treatment for ITO-Free Organic Solar Cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Lee, K.J.; Xiao, Y.; Woo, J.H.; Kim, E.; Kreher, D.; Attias, A.J.; Mathevet, F.; Ribierre, J.C.; Wu, J.W.; André, P. Charge-transfer dynamics and nonlocal dielectric permittivity tuned with metamaterial structures as solvent analogues. Nat. Mater. 2017, 16, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Krassas, M.; Kakavelakis, G.; Stylianakis, M.M.; Vaenas, N.; Stratakis, E.; Kymakis, E. Efficiency enhancement of organic photovoltaic devices by embedding uncapped Al nanoparticles in the hole transport layer. RSC Adv. 2015, 5, 71704–71708. [Google Scholar] [CrossRef]
- Kinder, R.; Mikolasek, M.; Donoval, D.; Kovac, J.; Tlaczala, M. Measurement system with hall and a four-point probes for characterization of semiconductors. J. Electr. Eng. 2013, 64, 106–111. [Google Scholar] [CrossRef]
- Ossila—Enabling Materials Science. Available online: https://www.ossila.com/ (accessed on 23 April 2019).
- Nicolaidis, N.C.; Routley, B.S.; Holdsworth, J.L.; Belcher, W.J.; Zhou, X.; Dastoor, P.C. Fullerene Contribution to Photocurrent Generation in Organic Photovoltaic Cells. J. Phys. Chem. C 2011, 115, 7801–7805. [Google Scholar] [CrossRef]
- Nicolaidis, N.C.; Al-Mudhaffer, M.F.; Holdsworth, J.L.; Zhou, X.; Belcher, W.J.; Dastoor, P.C. Contribution of Fullerene Photocurrent Generation to Organic Solar Cell Performance. J. Phys. Chem. C 2019, 123, 11950–11958. [Google Scholar] [CrossRef]

















| Structure | Name | Role in the Organic Solar Cell |
|---|---|---|
| In2O3/SnO2 | ITO | Anode electrode |
 | PEDOT:PSS | Hole transport/electron blocking layer |
 | PTB7 | Electron donor in the photoactive layer blend |
 | PC71BM | Electron acceptor in the photoactive layer blend |
| Ca | Calcium | Electron transport/hole blocking layer |
| Al | Aluminum | Cathode electrode |
 | MoO3 | Hole transport layer/hole mobility measurements |
 | PFN | Electron transport layer/electron mobility measurements |
| PC71BM | Donor Polymer | LUMO of Donor Polymer | HOMO of Donor Polymer | ΔELUMO | Egdonor |
| P3HT | −3 eV | −5.20 eV | 0.9 eV | 2 eV | |
| PCDTBT | −3.6 eV | −5.45 eV | 0.3 eV | 1.8 eV | |
| PTB7-Th | −3.66 eV | −5.24 eV | 0.24 eV | 1.58 eV | |
| PTB7 | −3.31 eV | −5.12 eV | 0.59 eV | 1.81 eV |
| PBT7 | Acceptor Material | LUMO of Acceptor Material | HOMO of Acceptor Material | ΔELUMO | Egacceptor |
| ICBA | −3.8 eV | −5.80 eV | 0.5 eV | 2 eV | |
| PDIs | −3.70–−4 eV | −5.70–−6 eV | 0.4–0.7 eV | 1.70–2.30 eV | |
| SubPcs | −3.50 eV | −5.70 eV | 0.4 eV | 2.20 eV |
| JSC (mA/cm2) | VOC (V) | FF (%) | PCE (%) | |
|---|---|---|---|---|
| Optimum Device | 16.32 | 0.76 | 59.6 | 7.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anagnostou, K.; Stylianakis, M.M.; Petridis, K.; Kymakis, E. Building an Organic Solar Cell: Fundamental Procedures for Device Fabrication. Energies 2019, 12, 2188. https://doi.org/10.3390/en12112188
Anagnostou K, Stylianakis MM, Petridis K, Kymakis E. Building an Organic Solar Cell: Fundamental Procedures for Device Fabrication. Energies. 2019; 12(11):2188. https://doi.org/10.3390/en12112188
Chicago/Turabian StyleAnagnostou, Katerina, Minas M. Stylianakis, Konstantinos Petridis, and Emmanuel Kymakis. 2019. "Building an Organic Solar Cell: Fundamental Procedures for Device Fabrication" Energies 12, no. 11: 2188. https://doi.org/10.3390/en12112188