1. Introduction
Lithium batteries have become the main source of power for electric vehicles because of the advantages they offer, such as reduced pollution, a long life cycle, high energy density, and good power performance [
1]. However, the performance of lithium batteries at low temperatures is poor. When the temperature decreases, the ohmic, polarization, and total internal resistance of batteries increase [
2]. For example, the ohmic resistance of a charging LiFePO
4 battery at −5 °C is five times that at room temperature [
3]. When the temperature is below −10 °C, there is a significant drop in battery capacity, as well as a loss in power [
4]. Battery charging is also more difficult than discharging in this environment. In this case, if the battery is forced to charge, lithium deposits and dendrites will appear on its negative electrode, which cause an internal short circuit [
5]. So far, it has been difficult to solve the low-temperature performance problem of lithium batteries through the use of innovative materials [
6]. Therefore, it is often necessary to heat the battery to a suitable operating temperature before using the battery in low temperature conditions.
At present, methods for heating batteries in low temperature environments are divided primarily into external heating and internal heating. Wang Facheng et al. [
7] used a heating wire to heat air at the inlet of an air duct of a battery box, and subsequently heat batteries through air convection. Hyun-Sik Song et al. [
8] also achieved battery heating by way of air convection. The above heating method can make the battery temperature rise rapidly to the appropriate temperature and the battery performance is improved significantly at low temperatures. However, this method causes unnecessary energy loss in the heating process, and the energy utilization of techniques that heat by way of air convection is low. Zhang Chengning et al. [
9] heat batteries using a wide-line metal film. Comparing with that it is almost not able to discharge prior to heating, the battery can subsequently release 50% of the stored electric energy after heating.
Liu Cunshan et al. [
10] established a low-temperature heating model for power batteries, and compared the effect of a positive temperature coefficient (PTC) heater and an electrothermal film heater. The electrothermal film heating mode does not affect the heat dissipation of the battery and has insulating performance at some degree. However, the power batteries used in electric vehicles are composed of a plurality of cells, which are arranged closely together, in series and in parallel [
11]. In the external heating mode, battery cells are not uniformly heated, which causes a rapid rise in local temperature. As a result, battery consistency deteriorates and the life of the battery pack is greatly shortened. In more severe cases, the deterioration in battery consistency causes failure of isolated cells, resulting in serious accidents. Compared to the external heating methods, the main advantage of internal heating is the use of heat generated by internal resistance in the charging/discharging process. The internal heating methods are characterized by high energy efficiency and can achieve uniform battery heating. Yan Ji et al. [
12] simulated a battery pack equivalent to two groups of cells, which, at a certain frequency, are alternately charged and discharged for battery heating after DC/DC boost, ultimately getting the ideal temperature rise effect. The mutual pulse heating consumes little battery power and is free of convective heat transfer system. However, it appears that the current used in this process is too large. In addition, the charging voltage of the battery in the heating process may reach 4.5 V, which is significantly higher than the charging cut-off voltage and increases the possibility of the formation of lithium dendrites. Zhang Jianbo et al. [
5] established a frequency domain model for a lithium-ion battery, which had a rated capacity of 3.1 A∙h, and proposed the use of sinusoidally alternating currents for internal heating. The battery can be heated from −20 °C to 5 °C within 15 min and the temperature distribution remains essentially uniform. However, the heating process is accompanied by large transient voltages. The maximum battery voltage recorded experimentally is 4.5 V. If an appropriate AC amplitude and frequency cannot be selected in practical applications, the battery may continue to be in a state of over-voltage, causing some damage. Zhao Xiaowei et al. [
13] proposed the use of a large current pulse for heating a 3.2 V, 12 A∙h lithium-iron phosphate battery. The charge and discharge cut-off voltages were 2.1 V and 3.6 V respectively. The heating process comprised a total of 18 charge and discharge cycles. In the final realization, the battery temperature rises from −10 °C to 3 °C. Ruan Haijun et al. [
14] heated batteries with a high-frequency alternating current, using a constant polarization voltage as a boundary condition. Ultimately, the battery temperature can be raised from −15 °C to 5.6 °C in 338 s. The constant polarization voltage is managed for battery heating to achieve a good tradeoff between short heating time and less damage to battery lifetime based on an electro-thermal coupled model. However, as the study only proved that there was no significant capacity decay in the battery after 30 repeated internal heating tests, the overall health of the battery, if the test is repeated more than 30 times, cannot be ascertained. Although pulsed heating can effectively heat batteries, alleviating the impact of low temperatures, larger charge pulse amplitudes result in stronger polarization of the anode surface, leading to the formation of lithium dendrites [
15].
The main reason for the failure of lithium batteries is the generation of lithium dendrites during the charging process in low-temperature environments [
16]. The lithium metal precipitates on the graphite anode surface at low temperatures or during charging at a high rate, and further reacts with the electrolyte. As a result, both available electrolyte and lithium ions are lost, and the battery volume changes, leading to poor contact between active substances and the current collector [
17]. The embedding of both electrolyte and lithium ions accelerates the peeling of graphite particles. The corrosion of both the collector and the adhesive reduces battery capacity [
18], eventually causing permanent damage to the battery. Though the discharging capacity of lithium batteries decreases and the discharging platform voltage drops, discharging in low temperature conditions does not cause permanent damage to the battery.
On the basis of the foregoing, this study develops a method to internally preheat lithium-ion batteries at low temperatures by way of constant-current discharging. This indicates that the temperature generated by internal resistance during battery discharging is used to heat the battery in a low temperature environment. Besides, it is difficult to predict the heating time and power consumption associated with the self-heating process of lithium-ion batteries at low temperatures. A temperature-rise model considering the dynamic changes in both battery temperature and state of charge (SOC) is thus proposed. When this model is combined with the ampere-hour integral method, the quantitative relationship among the discharge rate, heating time, and power consumption, during the progress of constant-current discharging for internally self-heating battery, is realized. Further, the problem of predicting the heating time and power consumption of the self-heating at low temperature is solved in this paper.
2. The Temperature-Rise Model
The Thevenin model is used to analyze the discharging process. As shown in
Figure 1,
represents the ohmic resistance,
is the voltage on
,
and
represents the polarization capacitance and polarization resistance respectively,
is the voltage on
and
,
is the open circuit voltage, E is the terminal voltage,
is the discharging current. In this paper,
is equivalent to the combination of
,
and
, which is annotated as
in the temperature-rise model.
Heat generated by a battery can be divided into irreversible heat and reversible heat. The irreversible heat includes Joule heat and concentration polarization heat. The reversible heat, also known as reaction heat, refers to energy that is released or absorbed in the electrochemical reaction to maintain the energy balance of the reaction. Referring to [
19], the simplified heat generation equation used in this paper can be expressed as (1):
where,
is the operating current of the battery (positive for charge, negative for discharge),
is the battery voltage,
is the open circuit voltage,
is the total heat generation power.
is the irreversible heat generation power, which represents the sum of both the heat generated by ohmic resistance when current flows and the heat generated by concentration difference through material transfer in the battery.
is the reversible entropic heat or reaction heat, which depends on the direction of current and the sign of the entropy coefficient. The entropy potential is greatly influenced by the state of charge (SOC) and varies with different chemical compositions [
20]. The difference between the battery terminal voltage and the open circuit voltage results from the voltage generated by internal resistance when current flows [
21]. Therefore, the irreversible heat can be expressed as Equation (2), where
is the equivalent internal resistance of the battery.
Battery temperature is influenced by heat generation, heat conduction, and thermal diffusion [
22]. In addition to internal heat production, the battery also distributes heat to the exterior when it works at a low temperatures. There are two main approaches for heat loss: convection and heat radiation. Thermal radiation is very small compared to thermal convection and is therefore ignored [
23]. The heat dissipation can be expressed by (3):
where
is the equivalent heat transfer coefficient,
is the surface area of the battery,
is the battery temperature, and
is the ambient temperature. Therefore, the heat balance equation can be obtained as the following equation:
where
is the mass of battery and
is the specific heat capacity. From Equation (4) we can see that the total heat generated by the battery is influenced by current, resistance, entropy potential, the equivalent heat transfer coefficient and battery temperature. One can yield that a greater current and resistance lead to greater heat generation. Conversely, a greater equivalent transfer coefficient and battery temperature results in more heat dissipation. As a result, the total heat generated is reduced. The battery temperature-rise model developed in this paper will take into account changes in the resistance and entropy coefficient during the process of battery heating so as to guarantee accuracy.
According to Equation (4), we can get the linear differential equation relating to battery temperature in Equation (5).
Equation (5) can be rewritten in discrete-time. The relevant expression, shown in Equation (6), is deduced, using the Laplace transform as,
where,
is the initial time and
is the current time. Under periodic sampling conditions,
,
, and
, Equation (6) can be rewritten as:
Upon further rearrangement, we can get Equation (8) as,
Equation (9) is obtained from Equation (8) by the inverse Laplace transform
4. Calculation Results and Analysis
According to the temperature-rise model developed in this paper, the time required for heating the battery from the ambient temperature to the target temperature at different discharge rates is obtained, as shown in
Figure 11. The curve is fitted by the least squares method to obtain the function of the battery discharge rate and the heating time, which is shown in Equation (12), where
is the discharge rate and
is the heating time in seconds.
As can be seen from
Figure 11, the battery temperature can be raised from −10 °C to 5 °C in 280 s when the discharge rate is 2 C. When the discharge rate decreases, the heating time gradually increases in response. The heating time is 1080 s when the discharge rate is 1 C. The effect of current discharge on the heating time is significantly enhanced when the discharge rate is less than 1 C. As the discharge rate continues to decrease, the heating time rapidly increases. The heating time is more than 2640 s when the discharge rate is 0.8 C, which is far longer than the reasonable heating time in actual applications.
Further, the power consumption of the self-heating process can be calculated by combining the battery temperature-rise model with the ampere-hour integral method [
29]. The ampere-hour integral equation is shown as Equation (17).
where
is the initial SOC of the battery,
is the SOC at time
,
is the discharge current of the battery, and
is the rated capacity of the battery. Additionally,
is defined as the power consumption in this paper. The power consumption of the battery during heating at different discharge current rates is shown in
Figure 12. The curve is fitted utilizing the least squares method to further obtain the function of the battery discharge rate and power consumption shown in Equation (18), where
is the discharge rate and
is the total variation of SOC during the heating process, i.e., the power consumption.
According to
Figure 12, the power consumption of the battery at a 2 C discharge rate is less than 15% of the rated capacity. As the discharge rate gradually reduced, the power consumption increases slowly. The power consumption of the heating process is 30% of the rated capacity when the discharge rate is 1 C. The effect of discharge rate on power consumption is significantly enhanced when it is less than 1 C. When the discharge rate is 0.8 C, the power consumption of the heating process is 60% of the rated capacity, which is twice the value at 1 C. Therefore, the discharge rate should be selected in the range of 1 C–2 C in applying the constant-current discharge method to heating a battery at low temperature.
5. Conclusions
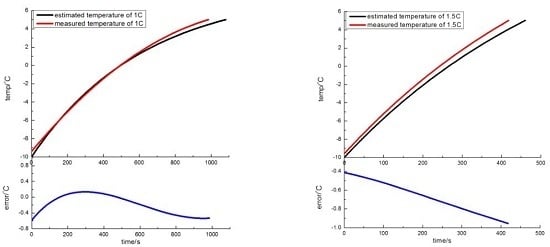
A temperature-rise model considering the dynamic fluctuation in battery temperature and SOC is proposed, and it is possible to predict the battery temperature during the progress of battery self-heating at low temperature. Tests in which the battery was heated from −10 °C to 5 °C were conducted at different discharge rates. The results show that the temperature-rise model can accurately reflect actual variation in battery temperature. The maximum error between the predicted temperature and actual temperature is less than 1 °C during the process of battery self-heating.
When the temperature-rise model developed in this paper is combined with the ampere-hour integral method, the quantitative relationship among the discharge rate, the heating time, and the power consumption during the self-heating process is realized. The difficulty in predicting the heating time and power consumption during the self-heating process is thus addressed. The results indicate that the discharge rate and the heating time present an exponential decreasing trend and it is similar with the discharge rate and the power consumption. When a 2 C discharge rate is selected for constant-current discharging to the internal heating battery, the battery temperature can rise from −10 °C to 5 °C in 280 s. In this case, the power consumption of the self-heating process does not exceed 15% of the rated capacity. As the discharge rate gradually reduced, the heating time and power consumption of the heating process increased slowly. When the discharge rate was 1 C, the heating time exceeded 1080 s, and the power consumption reached 30% of the rated capacity. The effect of discharge rate on the heating time and power consumption during the self-heating process is significantly enhanced when the discharge rate is less than 1 C. When the discharge rate is 0.8 C, the power consumption of self-heating process is 2.45 times that at 1 C, and the heating time is twice that at 1 C. Therefore, the discharge current rate should be selected in the range of 1 C–2 C in applying the constant-current discharge method to battery self-heating. The method of self-heating is suitable for heating the lithium-ion battery which is fully charged at low temperature before the normal operation.