A Simple Dip-Coating Method of SnO2-NiO Composite Thin Film on a Ceramic Tube Substrate for Methanol Sensing
Abstract
:1. Introduction
2. Materials and Methods
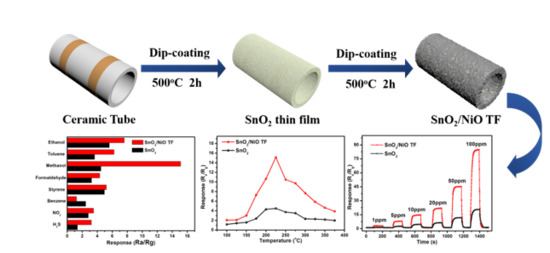
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choi, K.-I.; Hübner, M.; Haensch, A.; Kim, H.-J.; Weimar, U.; Barsan, N.; Jong, H.-L. Ambivalent effect of Ni loading on gas sensing performance in SnO2 based gas sensor. Sens. Actuators B Chem. 2013, 183, 401–410. [Google Scholar] [CrossRef]
- Ge, Q.; Ma, S.Y.; Xu, Y.B.; Xu, X.L.; Chen, H.; Qiang, Z.; Yang, H.M.; Ma, L.; Zeng, Z. Preparation, characterization and gas sensing properties of Pr-doped ZnO/SnO2 nanoflowers. Mater. Lett. 2017, 191, 5–9. [Google Scholar] [CrossRef]
- Rongjun, Z.; Xu, Z.; Sijia, P.; Ping, H.; Tong, Z.; Zidong, W.; Xinxin, X.; Yue, Y.; Yude, W. Shaddock peels as bio-templates synthesis of Cd-doped SnO2 nanofibers: A high performance formaldehyde sensing material. J. Alloy. Compd. 2020, 813, 152170. [Google Scholar]
- Park, K.-R.; Cho, H.-B.; Lee, J.; Song, Y.; Kim, W.-B.; Choa, Y.-H. Design of highly porous SnO2-CuO nanotubes for enhancing H2S gas sensor performance. Sens. Actuators B Chem. 2020, 302, 127179. [Google Scholar] [CrossRef]
- Yinglin, W.; Chang, L.; Lian, W.; Jie, L.; Bo, Z.; Yuan, G.; Peng, S.; Yanfeng, S.; Tong, Z.; Geyu, L. Horseshoe-shaped SnO2 with annulus-like mesoporous for ethanol gas sensing application. Sens. Actuators B Chem. 2017, 240, 1321–1329. [Google Scholar]
- Van Hieu, N.; Kim, H.-R.; Ju, B.-K.; Lee, J.-H. Enhanced performance of SnO2 nanowires ethanol sensor by functionalizing with La2O3. Sens. Actuators B Chem. 2008, 133, 228–234. [Google Scholar] [CrossRef]
- Kang, W.; Tianyu, Z.; Gang, L.; Qinqin, Y.; Chunhong, L.; Qilong, W.; Deliang, C. Room temperature CO sensor fabricated from Pt-loaded SnO2 porous nanosolid. Sens. Actuators B Chem. 2013, 184, 33–39. [Google Scholar] [CrossRef]
- Miao, Y.-E.; He, S.; Zhong, Y.; Yang, Z.; Tjiu, W.W.; Liu, T. A novel hydrogen peroxide sensor based on Ag/SnO2 composite nanotubes by electrospinning. Electrochim. Acta 2013, 99, 117–123. [Google Scholar] [CrossRef]
- Shouli, B.; Ke, T.; Hang, F.; Yongjun, F.; Ruixian, L.; Dianqing, L.; Aifan, C.; Chung, C.L. Novel α-Fe2O3/BiVO4 heterojunctions for enhancing NO2 sensing properties. Sens. Actuators B Chem. 2018, 268, 136–143. [Google Scholar]
- Shi, S.; Zhang, F.; Lin, H.; Wang, Q.; Shi, E.; Qu, F. Enhanced triethylamine-sensing properties of P-N heterojunction Co3O4/In2O3 hollow microtubes derived from metal–organic frameworks. Sens. Actuators B Chem. 2018, 262, 739–749. [Google Scholar] [CrossRef]
- Xiaoguang, S.; Guodong, Z.; Guosheng, W.; Yanbai, S.; Dan, M.; Yajing, Z.; Fanli, M. Assembly of 3D flower-like NiO hierarchical architectures by 2D nanosheets: Synthesis and their sensing properties to formaldehyde. RSC Adv. 2017, 7, 3540–3549. [Google Scholar]
- Jahromi, H.S.; Behzad, M. Construction of 0, 1, 2 and 3 dimensional SnO2 nanostructures decorated by NiO nanopetals: Structures, growth and gas-sensing properties. Mater. Chem. Phys. 2018, 207, 489–498. [Google Scholar] [CrossRef]
- Zhang, L.; He, J.; Jiao, W. Synthesis and gas sensing performance of NiO decorated SnO2 vertical-standing nanotubes composite thin films. Sens. Actuators B Chem. 2019, 281, 326–334. [Google Scholar] [CrossRef]
- Kun, L.; Yinzhen, W.; Mingpeng, C.; Qian, R.; Zhongqi, Z.; Qingju, L.; Jin, Z. High Methanol Gas-Sensing Performance of Sm2O3/ZnO/SmFeO3 Microspheres Synthesized Via a Hydrothermal Method. Nanoscale Res. Lett. 2019, 14, 57. [Google Scholar]
- Qing, L.; Tanyuan, W.; Dana, H.; Hanguang, Z.; Ping, X.; Jiantao, H.; Jaephil, C.; Gang, W. High-Performance Direct Methanol Fuel Cells with Precious-Metal-Free Cathode. Adv. Sci. 2016, 3, 1600140. [Google Scholar]
- Mengting, Z.; Yuxin, Y.; Chang, L.; Peiyi, H.; Song, H.; Liancheng, Z.; Xianshun, Z. A colorimetric and fluorometric dual-modal sensor for methanol based on a functionalized pentacenequinone derivative. Chem. Commun. 2018, 54, 8339–8342. [Google Scholar]
- Korotcenkov, G.; Brinzari, V.; Gulina, L.B.; Cho, B.K. The influence of gold nanoparticles on the conductivity response of SnO2-based thin film gas sensors. Appl. Surf. Sci. 2015, 353, 793–803. [Google Scholar] [CrossRef]
- Yadava, L.; Verma, R.; Dwivedi, R. Sensing properties of CdS-doped tin oxide thick film gas sensor. Sens. Actuators B Chem. 2010, 144, 37–42. [Google Scholar] [CrossRef]
- Liming, S.; Anatolii, L.; Denys, B.; Haibo, L.; Junkai, Z.; Ming, F.; Liying, L.; Duo, C.; Klyui, N.I. Facile Synthesis of Hierarchical Tin Oxide Nanoflowers with Ultra-High Methanol Gas Sensing at Low Working Temperature. Nanoscale Res. Lett. 2019, 14, 84. [Google Scholar]
- Li, Z.; Yi, J. Enhanced ethanol sensing of Ni-doped SnO2 hollow spheres synthesized by a one-pot hydrothermal method. Sens. Actuators B Chem. 2017, 243, 96–103. [Google Scholar] [CrossRef]
- Kim, J.; Mirzaei, H.A.; Kim, H.W.; Kim, S.S. Variation of shell thickness in ZnO-SnO2 core-shell nanowires for optimizing sensing behaviors to CO, C6H6, and C7H8 gases. Sens. Actuators B Chem. 2020, 302, 127150. [Google Scholar] [CrossRef]
- Sikai, Z.; Yanbai, S.; Pengfei, Z.; Jin, Z.; Wei, Z.; Xiangxiang, C.; Dezhou, W.; Ping, F.; Yansong, S. Highly selective NO2 sensor based on p-type nanocrystalline NiO thin films prepared by sol–gel dip coating. Ceram. Int. 2018, 44, 753–759. [Google Scholar]
- Xianghong, L.; Jun, Z.; Liwei, W.; Taili, Y.; Xianzhi, G.; Shihua, W.; Shurong, W. 3D hierarchically porous ZnO structures and their functionalization by Aunanoparticles for gas sensors. J. Mater. Chem. 2011, 21, 349–356. [Google Scholar]
- Li, C.C.; Yin, X.M.; Li, Q.H.; Wang, T.H. Enhanced gas sensing properties of ZnO/SnO2 hierarchical architectures by glucose-induced attachment. CrystEngComm 2011, 13, 1557–1563. [Google Scholar] [CrossRef]
- Tang, W.; Wang, J.; Yao, P.; Li, X. Hollow hierarchical SnO2-ZnO composite nanofibers with heterostructure based on electrospinning method for detecting methanol. Sens. Actuators B Chem. 2014, 192, 543–549. [Google Scholar] [CrossRef]
- Yang, H.M.; Ma, S.Y.; Yang, G.J.; Jin, W.X.; Wang, T.T.; Jiang, X.H.; Li, W.Q. High sensitive and low concentration detection of methanol by a gas sensor based on one-step synthesis α-Fe2O3 hollow spheres. Mater. Lett. 2016, 169, 73–76. [Google Scholar] [CrossRef]
- Tang, W. Sensing mechanism of SnO2/ZnO nanofibers for CH3OH sensors: Heterojunction effects. J. Phys. D Appl. Phys. 2017, 50, 475105. [Google Scholar] [CrossRef]
- Ji, H.-F.; Liu, W.-K.; Li, S.; Li, Y.; Shi, Z.-F.; Tian, T.Y.; Li, X.-J. High-performance methanol sensor based on GaN nanostructures grown on silicon nanoporous pillar array. Sens. Actuators B Chem. 2017, 250, 518–524. [Google Scholar] [CrossRef]
- Fang, W.; Hairong, L.; Zhaoxin, Y.; Yongzhe, S.; Fangzhi, C.; Heng, D.; Longzhen, X.; Haiyan, L. A highly sensitive gas sensor based on CuO nanoparticles synthetized via a sol–gel method. RSC Adv. 2016, 6, 79343–79349. [Google Scholar]
- Ding, X.; Zeng, D.; Xie, C. Controlled growth of SnO2 nanorods clusters via Zn doping and its influence on gas-sensing properties. Sens. Actuators B Chem. 2010, 149, 336–344. [Google Scholar] [CrossRef]
- Qin, J.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Liang, Y. Synthesis of three-dimensionally ordered macroporous LaFeO3 with enhanced methanol gas sensing properties. Sens. Actuators B Chem. 2015, 209, 706–713. [Google Scholar] [CrossRef]
- Wang, L.L.; Li, Z.J.; Luo, L.; Zhao, C.Z.; Kang, L.P.; Liu, D.W. Methanol sensing properties of honeycomb-like SnO2 grown on silicon nanoporous pillar array. J. Alloy. Compds. 2016, 682, 170–175. [Google Scholar] [CrossRef]
- Cai, Z.-X.; Li, H.-Y.; Ding, J.-C.; Guo, X. Hierarchical flowerlike WO3 nanostructures assembled by porous nanoflakes for enhanced NO gas sensing. Sens. Actuators B Chem. 2017, 246, 225–234. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Q.; Kou, C.; Sui, Y.; Zeng, Y.; Du, F. One-pot synthesis and improved sensing properties of hierarchical flowerlike SnO2 assembled from sheet and ultra-thin rod subunits. Sens. Actuators B Chem. 2014, 194, 447–453. [Google Scholar] [CrossRef]
- Li, L.; Yu, Z.; Guoguang, W.; Shouchun, L.; Lianyuan, W.; Yu, H.; Xiaoxue, J.; Aiguo, W. High toluene sensing properties of NiO-SnO2 composite nanofiber sensors operating at 330 °C. Sens. Actuators B Chem. 2011, 160, 448–454. [Google Scholar] [CrossRef]
- Geng, X.; Lahem, D.; Zhang, C.; Li, C.-J.; Olivier, M.-G.; Debliquy, M. Visible light enhanced black NiO sensors for ppb-level NO2 detection at room temperature. Ceram. Int. 2019, 45, 4253–4261. [Google Scholar] [CrossRef]
- Badlani, M.; Wachs, I.E. Methanol: A “Smart” Chemical Probe Molecule. Catal. Lett. 2001, 75, 137. [Google Scholar] [CrossRef]





| Sensing Material | Concentration (ppm) | Temperature (°C) | Response | Response/Recovery Time | Reference |
|---|---|---|---|---|---|
| Au decorated ZnO | 10 | 300 | 2.7 | ~20 s/~15 s | [23] |
| ZnO-SnO2 nanostructure microspheres | 10 | 300 | ~2 | ~18 s/~35 s | [24] |
| SnO2-ZnO composites nanofibers | 10 | 350 | 8.5 | 20 s/40 s | [25] |
| α-Fe2O3 hollow spheres | 10 | 280 | 25.1 | 8 s/9 s | [26] |
| SnO2/ZnO nanofibers | 10 | 350 | ~8.9 | ~12 s/~31 s | [27] |
| GaN nanostructures | 10 | 350 | ~1.3 | ~15 s/~30 s | [28] |
| CuO nanoparticles | 10 | 220 | 5.9 | 13 s/13 s | [29] |
| Zn doping SnO2 nanorods clusters | 10 | 270 | 11.5 | ~10 s/~10 s | [30] |
| three-dimensionally LaFeO3 | 10 | 190 | ~2 | ~12 s/~15 s | [31] |
| honeycomb-like SnO2 | 10 | 320 | ~4 | ~20 s/~20 s | [32] |
| SnO2/NiO thin film | 10 | 225 | 15.12 | 18 s/7 s | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Xu, L.; Wang, X.; Li, Q.; Cui, Q.; Suo, H.; Zhao, C. A Simple Dip-Coating Method of SnO2-NiO Composite Thin Film on a Ceramic Tube Substrate for Methanol Sensing. Crystals 2019, 9, 621. https://doi.org/10.3390/cryst9120621
Liu T, Xu L, Wang X, Li Q, Cui Q, Suo H, Zhao C. A Simple Dip-Coating Method of SnO2-NiO Composite Thin Film on a Ceramic Tube Substrate for Methanol Sensing. Crystals. 2019; 9(12):621. https://doi.org/10.3390/cryst9120621
Chicago/Turabian StyleLiu, Tianyu, Longxiao Xu, Xiangyue Wang, Qiuyang Li, Qiang Cui, Hui Suo, and Chun Zhao. 2019. "A Simple Dip-Coating Method of SnO2-NiO Composite Thin Film on a Ceramic Tube Substrate for Methanol Sensing" Crystals 9, no. 12: 621. https://doi.org/10.3390/cryst9120621