Advantages of Yolk Shell Catalysts for the DRM: A Comparison of Ni/ZnO@SiO2 vs. Ni/CeO2 and Ni/Al2O3
Abstract
:1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Catalyst Characterisation
2.3. Catalytic Activity
3. Results and Discussion
3.1. Textural Properties
3.2. XRD
3.3. SEM Analysis
3.4. Catalytic Activity
3.5. Temperature Screening
3.6. Stability Test
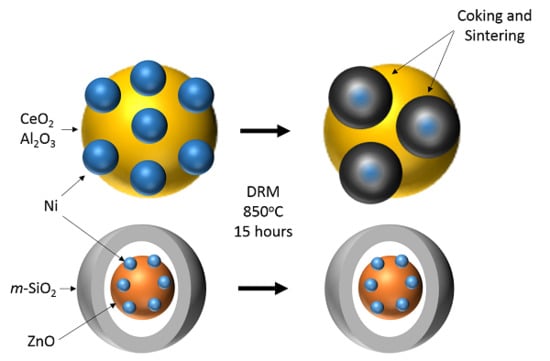
3.7. Post Reaction Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pires, J.C.M.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Simões, M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- European Commission. SETIS Magazine, 2016; 52.
- Dry, M.E. The Fischer-Tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Ganesh, I. Conversion of carbon dioxide into methanol—A potential liquid fuel: Fundamental challenges and opportunities (a review). Renew. Sustain. Energy Rev. 2014, 31, 221–257. [Google Scholar] [CrossRef]
- Kumar, N.; Shojaee, M.; Spivey, J.J. Catalytic bi-reforming of methane: From greenhouse gases to syngas. Curr. Opin. Chem. Eng. 2015, 9, 8–15. [Google Scholar] [CrossRef]
- Gronchi, P.; Centola, P.; Del Rosso, R. Dry reforming of CH4 with Ni and Rh metal catalysts supported on SiO2 and La2O3. Appl. Catal. A Gen. 1997, 152, 83–92. [Google Scholar] [CrossRef]
- Farsi, A.; Mansouri, S.S. Influence of nanocatalyst on oxidative coupling, steam and dry reforming of methane: A short review. Arab. J. Chem. 2016, 9, S28–S34. [Google Scholar] [CrossRef] [Green Version]
- Santos, B.A.V.; Loureiro, J.M.; Ribeiro, A.M.; Rodrigues, A.E.; Cunha, A.F. Methanol production by bi-reforming. Can. J. Chem. Eng. 2015, 93, 510–526. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mondal, K.C.; Choudhary, T. V Oxy-CO2 Reforming of Methane to Syngas over CoOx/MgO/SA-5205 catalyst. Fuel 2006, 20, 2–5. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Kaydouh, M.N.; El Hassan, N.; Davidson, A.; Casale, S.; El Zakhem, H.; Massiani, P. Effect of the order of Ni and Ce addition in SBA-15 on the activity in dry reforming of methane. C. R. Chim. 2015, 18, 293–301. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef] [Green Version]
- Behrens, M.; Armbrüster, M. Catalysis for Alternative Energy Generation—Methanol Steam Reforming. In Catalysis for Alternative Energy Generation; Springer: New York, NY, USA, 2012; pp. 175–235. ISBN 978-1-4614-0343-2. [Google Scholar]
- Rostrup-Nielsen, J.R. Industrial relevance of coking. Catal. Today 1997, 37, 225–232. [Google Scholar] [CrossRef]
- Guharoy, U.; Le Saché, E.; Cai, Q.; Reina, T.R.; Gu, S. Understanding the role of Ni-Sn interaction to design highly effective CO2 conversion catalysts for dry reforming of methane. J. CO2 Util. 2018, 27, 1–10. [Google Scholar] [CrossRef]
- Seo, H. Recent Scientific Progress on Developing Supported Ni Catalysts for Dry (CO2) Reforming of Methane. Catalysts 2018, 8, 110. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Avraam, D.G.; Goula, M.A. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Sehested, J.; Gelten, J.A.P.; Helveg, S. Sintering of nickel catalysts: Effects of time, atmosphere, temperature, nickel-carrier interactions, and dopants. Appl. Catal. A Gen. 2006, 309, 237–246. [Google Scholar] [CrossRef]
- Alotaibi, R.; Alenazey, F.; Alotaibi, F.; Wei, N.; Al-Fatesh, A.; Fakeeha, A. Ni catalysts with different promoters supported on zeolite for dry reforming of methane. Appl. Petrochem. Res. 2015, 5, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Valentini, A.; Carreño, N.L.V.; Probst, L.F.D.; Lisboa-Filho, P.N.; Schreiner, W.H.; Leite, E.R.; Longo, E. Role of vanadium in Ni:Al2O3 catalysts for carbon dioxide reforming of methane. Appl. Catal. A Gen. 2003, 255, 211–220. [Google Scholar] [CrossRef]
- Wang, M.; Boyjoo, Y.; Pan, J.; Wang, S.; Liu, J. Advanced yolk-shell nanoparticles as nanoreactors for energy conversion. Cuihua Xuebao/Chin. J. Catal. 2017, 38, 970–990. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Bian, Z.; Kathiraser, Y.; Kawi, S. Design of highly stable and selective core/yolk-shell nanocatalysts-review. Appl. Catal. B Environ. 2016, 188, 324–341. [Google Scholar] [CrossRef]
- Yang, W.; Liu, H.; Li, Y.; Zhang, J.; Wu, H.; He, D. Properties of yolk-shell structured Ni@SiO2 nanocatalyst and its catalytic performance in carbon dioxide reforming of methane to syngas. Catal. Today 2016, 259, 438–445. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Shi, W. Syngas production from CO2 reforming with methane over core-shell Ni@SiO2 catalysts. J. CO2 Util. 2016, 16, 318–327. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.H.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Silica–Ceria sandwiched Ni core–shell catalyst for low temperature dry reforming of biogas: Coke resistance and mechanistic insights. Appl. Catal. B Environ. 2018, 230, 220–236. [Google Scholar] [CrossRef]
- Zhao, X.; Li, H.; Zhang, J.; Shi, L.; Zhang, D. Design and synthesis of NiCe@m-SiO2 yolk-shell framework catalysts with improved coke- and sintering-resistance in dry reforming of methane. Int. J. Hydrogen Energy 2016, 41, 2447–2456. [Google Scholar] [CrossRef]
- Damyanova, S.; Bueno, J.M.C. Effect of CeO2 loading on the surface and catalytic behaviors of CeO2-Al2O3-supported Pt catalysts. Appl. Catal. A Gen. 2003, 253, 135–150. [Google Scholar] [CrossRef]
- Verykios, X.E. Mechanistic aspects of the reaction of CO2 reforming of methane over Rh/Al2O3 catalyst. Appl. Catal. A Gen. 2003, 255, 101–111. [Google Scholar] [CrossRef]
- Pines, H.; Haag, W.O. Alumina: Catalyst and Support. I. Alumina, Its Intrinsic Acidity and Catalytic Activity. J. Am. Chem. Soc. 1960, 82, 2471–2483. [Google Scholar] [CrossRef]
- Stroud, T.; Smith, T.J.; Le Saché, E.; Santos, J.L.; Centeno, M.A.; Arellano-Garcia, H.; Odriozola, J.A.; Reina, T.R. Chemical CO2 recycling via dry and bi reforming of methane using Ni-Sn/Al2O3 and Ni-Sn/CeO2-Al2O3 catalysts. Appl. Catal. B Environ. 2018, 224, 125–135. [Google Scholar] [CrossRef]
- Djinović, P.; Batista, J.; Pintar, A. Efficient catalytic abatement of greenhouse gases: Methane reforming with CO2 using a novel and thermally stable Rh-CeO2 catalyst. Int. J. Hydrogen Energy 2012, 37, 2699–2707. [Google Scholar] [CrossRef]
- Le Saché, E.; Pastor-Pérez, L.; Watson, D.; Sepúlveda-Escribano, A.; Reina, T.R. Ni stabilised on inorganic complex structures: Superior catalysts for chemical CO2 recycling via dry reforming of methane. Appl. Catal. B Environ. 2018, 236, 458–465. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Chen, J.S.; Lou, X.W.; Xing, X.; Lu, G.Q. Yolk/shell nanoparticles: New platforms for nanoreactors, drug delivery and lithium-ion batteries. Chem. Commun. 2011, 47, 12578. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Ai, G.; Zhang, K.; Yuan, Q.; Han, Y.; Ma, X.; Wang, J.; Liu, S. Coking resistant Ni/ZrO2@SiO2 catalyst for the partial oxidation of methane to synthesis gas. Int. J. Hydrogen Energy 2015, 40, 6835–6843. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F. Coke-resistant Ni at SiO2 catalyst for dry reforming of methane. Appl. Catal. B Environ. 2015, 176–177, 513–521. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.Y.; Han, S.W.; Cho, Y.K.; Jeong, M.G.; Park, E.J.; Kim, Y.D. The catalytic stability of TiO2-shell/Ni-core catalysts for CO2 reforming of CH4. Appl. Catal. A Gen. 2015, 495, 184–191. [Google Scholar] [CrossRef]
- Baktash, E.; Littlewood, P.; Schomäcker, R.; Thomas, A.; Stair, P.C. Alumina coated nickel nanoparticles as a highly active catalyst for dry reforming of methane. Appl. Catal. B Environ. 2015, 179, 122–127. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Lo Mastro, S.; Sodo, A. Ni/CeO2-Al2O3 catalysts for the dry reforming of methane: The effect of CeAlO3 content and nickel crystallite size on catalytic activity and coke resistance. Appl. Catal. A Gen. 2015, 500, 12–22. [Google Scholar] [CrossRef]
- Ay, H.; Üner, D. Dry reforming of methane over CeO2 supported Ni, Co and Ni-Co catalysts. Appl. Catal. B Environ. 2015, 179, 128–138. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kent, R.A.; Yung, M.M.; Kuhn, J.N. Carbon dioxide conversion by reverse water-gas shift chemical looping on perovskite-type oxides. Ind. Eng. Chem. Res. 2014, 53, 5828–5837. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic dry reforming of methane over high surface area ceria. Appl. Catal. B Environ. 2005, 60, 107–116. [Google Scholar] [CrossRef]
- Kovacevic, M.; Mojet, B.L.; Van Ommen, J.G.; Lefferts, L. Effects of Morphology of Cerium Oxide Catalysts for Reverse Water Gas Shift Reaction. Catal. Lett. 2016, 146, 770–777. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, B.; Abd Ghani, N.A.; Vo, D.V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef]
- De Falco, M.; Iaquaniello, G.; Centi, G. CO2: A valuable source of carbon. Green Energy Technol. 2013, 137, 194. [Google Scholar] [CrossRef]
- Bereketidou, O.A.; Goula, M.A. Biogas reforming for syngas production over nickel supported on ceria-alumina catalysts. Catal. Today 2012, 195, 93–100. [Google Scholar] [CrossRef]
- Hou, Z.; Yokota, O.; Tanaka, T.; Yashima, T. Characterization of Ca-promoted Ni/α-Al2O3 catalyst for CH4 reforming with CO2. Appl. Catal. A Gen. 2003, 253, 381–387. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Sophiphun, O.; Bernardi, J.; Wittayakun, J.; Föttinger, K.; Rupprechter, G. Methane dry reforming over ceria-zirconia supported Ni catalysts. Catal. Today 2016, 277, 234–245. [Google Scholar] [CrossRef]
- Seok, S.H.; Sun, H.C.; Park, E.D.; Sung, H.H.; Jae, S.L. Mn-promoted Ni/Al2O3 catalysts for stable carbon dioxide reforming of methane. J. Catal. 2002, 209, 6–15. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.L.; Couzi, M. Raman spectroscopy as a tool for the analysis of carbon-based materials (highly oriented pyrolitic graphite, multilayer graphene and multiwall carbon nanotubes) and of some of their elastomeric composites. Vib. Spectrosc. 2014, 74, 57–63. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.A.; Park, W.; Cao, H.; Chena, Y.P. Raman Spectroscopy of Graphene and Related Materials. Front. Mol. Spectrosc. 2009, 553–595. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon N. Y. 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Price, C.; Pastor-Pérez, L.; le Saché, E.; Sepúlveda-Escribano, A.; Reina, T.R. Highly active Cu-ZnO catalysts for the WGS reaction at medium–high space velocities: Effect of the support composition. Int. J. Hydrogen Energy 2017, 42, 10747–10751. [Google Scholar] [CrossRef] [Green Version]







| Sample | SBET (m2g−1) | Vpore (cm3g−1) | DPore (nm) |
|---|---|---|---|
| Ni/Al2O3 | 158 | 0.40 | 6.8 |
| Ni/CeO2 | 81 | 0.06 | 3.3 |
| Ni–ZnO@SiO2 | 700 | 0.55 | 2.6 |
| Sample | R550 CH4 (molconv.s−1/moleNi) | R650 CH4 (molconv.s−1/moleNi) | R800 CH4 (molconv.s−1/moleNi) | R550 CO2 (molconv.s−1/moleNi) | R650 CO2 (molconv.s−1/moleNi) | R800 CO2 (molconv.s−1/moleNi) |
|---|---|---|---|---|---|---|
| Ni–ZnO@SiO2 | 1.09 | 2.24 | 4.56 | 1.64 | 3.18 | 5.55 |
| Ni/CeO2 | 0.42 | 1.21 | 2.89 | 0.42 | 1.99 | 3.98 |
| Ni/Al2O3 | 0.84 | 1.03 | 1.33 | 1.22 | 1.43 | 1.78 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Price, C.A.H.; Earles, E.; Pastor-Pérez, L.; Liu, J.; Reina, T.R. Advantages of Yolk Shell Catalysts for the DRM: A Comparison of Ni/ZnO@SiO2 vs. Ni/CeO2 and Ni/Al2O3. Chemistry 2019, 1, 3-16. https://doi.org/10.3390/chemistry1010003
Price CAH, Earles E, Pastor-Pérez L, Liu J, Reina TR. Advantages of Yolk Shell Catalysts for the DRM: A Comparison of Ni/ZnO@SiO2 vs. Ni/CeO2 and Ni/Al2O3. Chemistry. 2019; 1(1):3-16. https://doi.org/10.3390/chemistry1010003
Chicago/Turabian StylePrice, Cameron Alexander Hurd, Emily Earles, Laura Pastor-Pérez, Jian Liu, and Tomas Ramirez Reina. 2019. "Advantages of Yolk Shell Catalysts for the DRM: A Comparison of Ni/ZnO@SiO2 vs. Ni/CeO2 and Ni/Al2O3" Chemistry 1, no. 1: 3-16. https://doi.org/10.3390/chemistry1010003