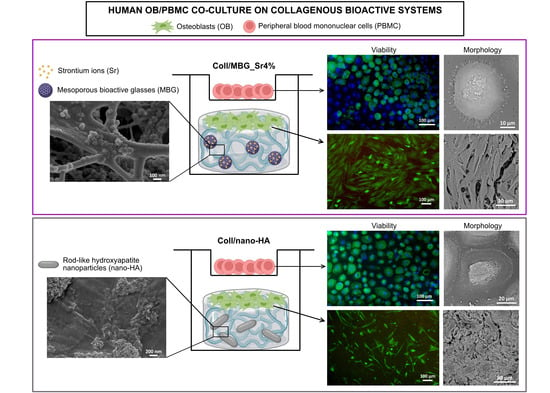
Assessment of Collagen-Based Nanostructured Biomimetic Systems with a Co-Culture of Human Bone-Derived Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Collagen-Based Composite Systems (Coll/MBG_ Sr4% and Coll/Nano-HA)
2.2. Characterization of the Collagen-Based Composite Systems
2.2.1. Morphological and Physico-Chemical Assessment by Means of Field Emission Scanning Electron Microscopy (FESEM)
2.2.2. Rheological Properties
2.2.3. In Vitro Enzymatic and Hydrolytic Degradation
2.3. In Vitro Biological Assessment: Indirect Co-Culture System
2.3.1. Osteoblasts Isolation and Culture
2.3.2. Peripheral Blood Mononuclear Cell Isolation and Culture
2.3.3. Indirect Co-Culture Set Up with Coll/MG_Sr4% and Coll/Nano-HA Systems
2.3.4. Assessment of Cell Viability
2.3.5. Indirect Cytotoxicity Test
2.3.6. Cell Morphology with Scanning Electron Microscopy (SEM)
2.3.7. Alkaline Phosphatase (ALP) Activity
2.3.8. Assessment of Osteoclast Precursor Maturation
2.4. Statistical Analysis
3. Results
3.1. Physico-Chemical and Structural Properties of the Collagen-Based Biomimetic Systems
3.2. Biological Assessment of Indirect Co-Culture
3.2.1. Cell Viability with Alamar Blue
3.2.2. Cell Viability with Live Dead Assay
3.2.3. Indirect Cytotoxicity Test
3.2.4. Cell Morphology with SEM
3.2.5. Alkaline Phosphatase (ALP) Activity
3.2.6. Assessment of Osteoclast Precursor Maturation
4. Discussion
4.1. Implementation of the Co-Culture Method and Material’s Suitability
4.2. Cell Viability and Proliferation in Direct and Indirect Contact with the Materials
4.3. Effect of the Biomaterials on Cell Morphology and Differentiation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sozen, T.; Ozisik, L.; Calik Basaran, N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A review of treatment options. Pharm. Ther. 2018, 43, 92–104. [Google Scholar]
- Matsumoto, T.; Fukumoto, S. Recent advances in the management of osteoporosis. F1000Research 2017, 6, 625. [Google Scholar] [CrossRef] [Green Version]
- Masi, L. Epidemiology of osteoporosis. Clin. Cases Miner. Bone Metab. 2008, 5, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Akkawi, I.; Zmerly, H. Osteoporosis: Current concepts. Joints 2018, 6, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, C.; Melton, L.J., III. Epidemiology of osteoporosis. Trends Endocrinol. Metab. 1992, 3, 224–229. [Google Scholar] [CrossRef]
- Pazianas, M.; Van Der Geest, S.; Miller, P. Bisphosphonates and bone quality. Bonekey Rep. 2014, 3, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapi, F.; Cipriani, F.; Caputi, A.P.; Corrao, G.; Vaccheri, A.; Sturkenboom, M.C.; Di Bari, M.; Gregori, D.; Carle, F.; Staniscia, T.; et al. Assessing the risk of osteonecrosis of the jaw due to bisphosphonate therapy in the secondary prevention of osteoporotic fractures. Osteoporos. Int. 2013, 24, 697–705. [Google Scholar] [CrossRef]
- Misof, B.M.; Fratzl-Zelman, N.; Paschalis, E.P.; Roschger, P.; Klaushofer, K. Long-term safety of antiresorptive treatment: Bone material, matrix and mineralization aspects. Bonekey Rep. 2015, 4, 634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.P.; Morin, S.; Leslie, W.; Papaioannou, A.; Cheung, A.M.; Davison, K.S.; Goltzman, D.; Hanley, D.A.; Hodsman, A.; Josse, R.; et al. Bisphosphonates for treatment of osteoporosis. Expected benefits, potential harms, and drug holidays. Can. Fam. Physician 2014, 60, 324–333. [Google Scholar] [PubMed]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, B.D.; Stegemann, J.P. Strategies for directing the structure and function of 3D collagen biomaterials across length scales. Acta Biomater. 2014, 10, 1488–1501. [Google Scholar] [CrossRef] [Green Version]
- Borciani, G.; Montalbano, G.; Baldini, N.; Cerqueni, G.; Vitale-brovarone, C.; Ciapetti, G. Co-culture systems of osteoblasts and osteoclasts: Simulating in vitro bone remodeling in regenerative approaches. Acta Biomater. 2020, 108, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Seeman, E. Age- and Menopause-Related Bone Loss Compromise Cortical and Trabecular Microstructure. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2013, 68, 1218–1225. [Google Scholar] [CrossRef] [Green Version]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 25. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Lee, J.H.; Lee, E.J.; Kim, H.W. Collagen hydrogels incorporated with surface-aminated mesoporous nanobioactive glass: Improvement of physicochemical stability and mechanical properties is effective for hard tissue engineering. Acta Biomater. 2013, 9, 9392–9400. [Google Scholar] [CrossRef] [PubMed]
- Sarker, B.; Hum, J.; Nazhat, S.N.; Boccaccini, A.R. Combining collagen and bioactive glasses for bone tissue engineering: A review. Adv. Healthc. Mater. 2015, 4, 176–194. [Google Scholar] [CrossRef]
- Kołodziejska, B.; Kaflak, A.; Kolmas, J. Biologically Inspired Collagen/Apatite Composite Biomaterials for Potential Use in Bone Tissue Regeneration—A Review. Materials 2020, 13, 1748. [Google Scholar] [CrossRef] [Green Version]
- Montalbano, G.; Fiorilli, S.; Caneschi, A.; Vitale-Brovarone, C. Type I collagen and strontium-containing mesoporous glass particles as hybrid material for 3D printing of bone-like materials. Materials 2018, 11, 700. [Google Scholar] [CrossRef] [Green Version]
- Montalbano, G.; Borciani, G.; Cerqueni, G.; Licini, C.; Banche-Niclot, F.; Janner, D.; Sola, S.; Fiorilli, S.; Mattioli-Belmonte, M.; Ciapetti, G.; et al. Collagen hybrid formulations for the 3d printing of nanostructured bone scaffolds: An optimized genipin-crosslinking strategy. Nanomaterials 2020, 10, 1681. [Google Scholar] [CrossRef]
- Kyllönen, L.; D’Este, M.; Alini, M.; Eglin, D. Local drug delivery for enhancing fracture healing in osteoporotic bone. Acta Biomater. 2015, 11, 412–434. [Google Scholar] [CrossRef] [PubMed]
- Delaisse, J.-M. The reversal phase of the bone-remodeling cycle: Cellular prerequisites for coupling resorption and formation. Bonekey Rep. 2014, 3, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast–osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, G.; Molino, G.; Fiorilli, S.; Vitale-Brovarone, C. Synthesis and incorporation of rod-like nano-hydroxyapatite into type I collagen matrix: A hybrid formulation for 3D printing of bone scaffolds. J. Eur. Ceram. Soc. 2020, 40, 3689–3697. [Google Scholar] [CrossRef]
- Montalbano, G.; Borciani, G.; Pontremoli, C.; Ciapetti, G.; Mattioli-Belmonte, M.; Fiorilli, S.; Vitale-Brovarone, C. Development and Biocompatibility of Collagen-Based Composites Enriched with Nanoparticles of Strontium Containing Mesoporous Glass. Materials 2019, 12, 3719. [Google Scholar] [CrossRef] [Green Version]
- Schwarcz, H.P.; Abueidda, D.; Jasiuk, I. The Ultrastructure of Bone and Its Relevance to Mechanical Properties. Front. Phys. 2017, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Griffanti, G.; Rezabeigi, E.; Li, J.; Murshed, M.; Nazhat, S.N. Rapid Biofabrication of Printable Dense Collagen Bioinks of Tunable Properties. Adv. Funct. Mater. 2020, 30, 1903874. [Google Scholar] [CrossRef]
- Ficai, A.; Andronescu, E.; Ghitulica, C.; Voicu, G.; Trandafir, V.; Manzu, D.; Ficai, M.; Pall, S. Colagen/hydroxyapatite interactions in composite biomaterials. Mater. Plast. 2009, 46, 11–15. [Google Scholar]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng.-Part B 2017, 23, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Halai, M.; Ker, A.; Meek, R.D.; Nadeem, D.; Sjostrom, T.; Su, B.; McNamara, L.E.; Dalby, M.J.; Young, P.S. Scanning electron microscopical observation of an osteoblast/osteoclast co-culture on micropatterned orthopaedic ceramics. J. Tissue Eng. 2014, 5, 2041731414552114. [Google Scholar] [CrossRef]
- Janardhanan, S.; Wang, M.O.; Fisher, J.P. Coculture Strategies in Bone Tissue Engineering: The Impact of Culture Conditions on Pluripotent Stem Cell Populations. Tissue Eng. Part B Rev. 2012, 18, 312–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhardt, A.; Thieme, S.; Domaschke, H.; Springer, A.; Rösen-Wolff, A.; Gelinsky, M. Crosstalk of osteoblast and osteoclast precursors on mineralized collagen-towards an in vitro model for bone remodeling. J. Biomed. Mater. Res.-Part A 2010, 95, 848–856. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- Da, W.; Tao, L.; Zhu, Y. The Role of Osteoclast Energy Metabolism in the Occurrence and Development of Osteoporosis. Front. Endocrinol. 2021, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Lemma, S.; Sboarina, M.; Porporato, P.E.; Zini, N.; Sonveaux, P.; Di Pompo, G.; Baldini, N.; Avnet, S. Energy metabolism in osteoclast formation and activity. Int. J. Biochem. Cell Biol. 2016, 79, 168–180. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Chi, G.; Li, P.; Lv, S.; Xu, J.; Xu, Z.; Xia, Y.; Tan, Y.; Xu, J.; Li, L.; et al. Effects of Matrix Stiffness on the Morphology, Adhesion, Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Med. Sci. 2018, 15, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, K.; Lin-Gibson, S.; Wallace, W.E.; Parekh, S.H.; Lee, Y.J.; Cicerone, M.T.; Young, M.F.; Simon, C.G. The effect of 3D hydrogel scaffold modulus on osteoblast differentiation and mineralization revealed by combinatorial screening. Biomaterials 2010, 31, 5051–5062. [Google Scholar] [CrossRef] [Green Version]
- Hatakeyama, w.; Taira, m.; Chosa, n.; Kihara, h.; Ishisaki, a.; Kondo, h. Effects of apatite particle size in two apatite/collagen composites on the osteogenic differentiation profile of osteoblastic cells. Int. J. Mol. Med. 2013, 32, 1255–1261. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Huang, X.; Cai, Y.; Tang, R.; Yang, D. Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater. 2009, 5, 338–345. [Google Scholar] [CrossRef]
- Ha, S.-W.; Jang, H.L.; Nam, K.T.; Beck, G.R. Nano-hydroxyapatite modulates osteoblast lineage commitment by stimulation of DNA methylation and regulation of gene expression. Biomaterials 2015, 65, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, J.M.; Molinuevo, M.S.; McCarthy, A.D.; Cortizo, A.M. Strontium ranelate stimulates the activity of bone-specific alkaline phosphatase: Interaction with Zn2+ and Mg2+. BioMetals 2014, 27, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Fiorilli, S.; Molino, G.; Pontremoli, C.; Iviglia, G.; Torre, E.; Cassinelli, C.; Morra, M.; Vitale-Brovarone, C. The Incorporation of Strontium to Improve Bone-Regeneration Ability of Mesoporous Bioactive Glasses. Materials 2018, 11, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jell, G.; Stevens, M.M. Gene activation by bioactive glasses. Mater. Sci. Mater. Med. 2006, 17, 997–1002. [Google Scholar] [CrossRef]
- Wu, T.; Cheng, N.; Xu, C.; Sun, W.; Yu, C.; Shi, B. The effect of mesoporous bioglass on osteogenesis and adipogenesis of osteoporotic BMSCs. J. Biomed. Mater. Res. Part A 2016, 104, 3004–3014. [Google Scholar] [CrossRef] [Green Version]
- Taherkhani, S.; Moztarzadeh, F. Influence of strontium on the structure and biological properties of sol–gel-derived mesoporous bioactive glass (MBG) powder. J. Sol-Gel Sci. Technol. 2016, 78, 539–549. [Google Scholar] [CrossRef]
- Baron, R.; Tsouderos, Y. In vitro effects of S12911-2 on osteoclast function and bone marrow macrophage differentiation. Eur. J. Pharmacol. 2002, 450, 11–17. [Google Scholar] [CrossRef]
- Lakkakorpi, P.T.; Väänänen, H.K. Cytoskeletal changes in osteoclasts during the resorption cycle. Microsc. Res. Tech. 1996, 33, 171–181. [Google Scholar] [CrossRef]
- Sun, T.; Li, Z.; Zhong, X.; Cai, Z.; Ning, Z.; Hou, T.; Xiong, L.; Feng, Y.; Leung, F.; Lu, W.W.; et al. Strontium inhibits osteoclastogenesis by enhancing LRP6 and β-catenin-mediated OPG targeted by miR-181d-5p. J. Cell Commun. Signal. 2019, 13, 85–97. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borciani, G.; Montalbano, G.; Melo, P.; Baldini, N.; Ciapetti, G.; Vitale-Brovarone, C. Assessment of Collagen-Based Nanostructured Biomimetic Systems with a Co-Culture of Human Bone-Derived Cells. Cells 2022, 11, 26. https://doi.org/10.3390/cells11010026
Borciani G, Montalbano G, Melo P, Baldini N, Ciapetti G, Vitale-Brovarone C. Assessment of Collagen-Based Nanostructured Biomimetic Systems with a Co-Culture of Human Bone-Derived Cells. Cells. 2022; 11(1):26. https://doi.org/10.3390/cells11010026
Chicago/Turabian StyleBorciani, Giorgia, Giorgia Montalbano, Priscila Melo, Nicola Baldini, Gabriela Ciapetti, and Chiara Vitale-Brovarone. 2022. "Assessment of Collagen-Based Nanostructured Biomimetic Systems with a Co-Culture of Human Bone-Derived Cells" Cells 11, no. 1: 26. https://doi.org/10.3390/cells11010026