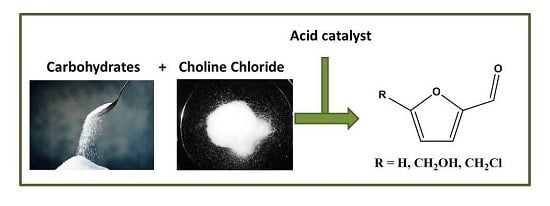
Catalytic Conversion of Carbohydrates to Furanic Derivatives in the Presence of Choline Chloride
Abstract
:1. Introduction
2. Synthesis of Furanic Derivatives in the Presence of ChCl (LMM) and a Catalyst
2.1. Lewis Acids as Catalysts
2.2. Brønsted Acids as Catalysts
2.3. Heteropolyacids as Catalysts
3. Synthesis of Furanic Derivatives in the Presence of a DES Composed of ChCl and a Carboxylic Acid
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Ståhlberg, T.; Fu, W.J.; Woodley, J.M.; Riisager, A. Synthesis of 5-(hydroxymethyl)furfural in ionic liquids: Paving the way to renewable chemicals. ChemSusChem 2011, 4, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural—A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Teong, S.P.; Yi, G.; Zhang, Y. Hydroxymethylfurfural production from bioresources: Past, present and future. Green Chem. 2014, 16, 2015–2020. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Ruß, C.; König, B. Low melting mixtures in organic synthesis—An alternative to ionic liquids? Green Chem. 2012, 14, 2969–2982. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Carriazo, D.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Deep-eutectic solvents playing multiple roles in the synthesis of polymers and related materials. Chem. Soc. Rev. 2012, 41, 4996–5014. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-Transition-Temperature Mixtures (LTTMs): A New Generation of Designer Solvents. Ang. Chem. Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Del Monte, F.; Carriazo, D.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L. Deep Eutectic Solvents in Polymerizations: A Greener Alternative to Conventional Syntheses. ChemSusChem 2014, 7, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Namieśnik, J. Ionic Liquids and Deep Eutectic Mixtures: Sustainable Solvents for Extraction Processes. ChemSusChem 2014, 7, 1784–1800. [Google Scholar] [CrossRef] [PubMed]
- Wagle, D.V.; Zhao, H.; Baker, G.A. Deep Eutectic Solvents: Sustainable Media for Nanoscale and Functional Materials. Acc. Chem. Res. 2014, 47, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Hao, J.; Mo, L.; Zhang, Z. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv. 2015, 5, 48675–48704. [Google Scholar] [CrossRef]
- Ilgen, F.; Ott, D.; Kralish, D.; Reil, C.; Palmberger, A.; König, B. Conversion of carbohydrates into 5-hydroxymethylfurfural in highly concentrated low melting mixtures. Green Chem. 2009, 11, 1948–1954. [Google Scholar] [CrossRef]
- Liu, F.; Audemar, M.; De Oliveira Vigier, K.; Cartigny, D.; Clacens, J.-M.; Costa Gomes, M.F.; Pádua, A.A.H.; De Campo, F.; Jérôme, F. Selectivity enhancement in the aqueous acid-catalyzed conversion of glucose to 5-hydroxymethylfurfural induced by choline chloride. Green Chem. 2013, 15, 3205–3213. [Google Scholar] [CrossRef]
- Yang, J.; De Oliveira Vigier, K.; Gu, Y.; Jérôme, F. Catalytic dehydration of carbohydrates suspended in organic solvents promoted by AlCl3/SiO2 coated with choline chloride. ChemSusChem 2015, 3, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.; Li, Z.; Jiang, Y.; Tang, X.; Zeng, X.; Sun, Y.; Lin, L. Green catalytic conversion of bio-based sugars to 5-chloromethyl furfural in deep eutectic solvent catalyzed by metal chlorides. RSC Adv. 2016, 6, 27004–27007. [Google Scholar] [CrossRef]
- Assanosi, A.A.; Farah, M.M.; Wood, J.; Al-Duri, B. A facile acidic choline chloride–p-TSA DES catalysed dehydration of fructose to 5-hydroxymethylfurfural. RSC Adv. 2014, 4, 39359–39364. [Google Scholar] [CrossRef]
- Liu, F.; Barrault, J.; De Oliveira Vigier, K.; Jérôme, F. Dehydration of highly concentrated solution of fructose to 5-hydroxymethylfurfural in cheap and sustainable choline chloride/CO2 system. ChemSusChem 2012, 5, 1223–1226. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hou, M.; Han, B.; Wang, X.; Zou, L. Solubility of CO2 in a Choline Chloride + Urea Eutectic Mixture. J. Chem. Eng. Data 2008, 53, 548–550. [Google Scholar] [CrossRef]
- De Oliveira Vigier, K.; Benguerba, A.; Barrault, J.; Jérôme, F. Conversion of fructose and inulin to 5-hydroxymethylfurfural in sustainable betaine hydrochloride-based media. Green Chem. 2012, 14, 285–289. [Google Scholar] [CrossRef]
- Mondal, D.; Chaudhary, J.P.; Sharma, M.; Prasad, K. Simultaneous dehydration of biomass-derived sugars to 5-hydroxymethyl furfural (HMF) and reduction of graphene oxide in ethyl lactate: One pot dual chemistry. RSC Adv. 2014, 4, 29834–29839. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, Z.; Wang, S.; Huang, G.; Wang, X.; Jiang, Z. Conversion of highly concentrated fructose into 5-hydroxymethylfurfural by acid–base bifunctional HPA nanocatalysts induced by choline chloride. RSC Adv. 2014, 4, 63055–63061. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Sun, Z.; Xue, L.; Wang, X.; Jiang, Z. Highly efficient preparation of HMF from cellulose using temperature-responsive heteropolyacid catalysts in cascade reaction. Appl. Catal. B 2016, 196, 50–56. [Google Scholar] [CrossRef]
- Ghezali, W.; De Oliveira Vigier, K.; Kessas, R.; Jérôme, F. A choline chloride/DMSO solvent for the direct synthesis of diformylfuran from carbohydrates in the presence of heteropolyacids. Green Chem. 2015, 17, 4459–4464. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Zhou, Y.; Han, B.; Fan, H.; Li, W.; Song, J.; Xie, Y. Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials. Green Chem. 2008, 10, 1280–1283. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Zhou, Y.; Song, J.; Fan, H.; Han, B. Direct conversion of inulin to 5-hydroxymethylfurfural in biorenewable ionic liquids. Green Chem. 2009, 11, 873–877. [Google Scholar] [CrossRef]
- Matsumiya, H.; Hara, T. Conversion of glucose into 5-hydroxymethylfurfural with boric acid in molten mixtures of choline salts and carboxylic acids. Biomass Bioenergy 2015, 72, 227–232. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H. Conversion of xylan and xylose into furfural in biorenewable deep eutectic solvent with trivalent metal chloride added. Bioresources 2013, 8, 6014–6025. [Google Scholar] [CrossRef]
- Da Silva Lacerda, V.; López-Sotelo, J.B.; Correa-Guimarães, A.; Hernández-Navarro, S.; Sánchez-Bascones, M.; Navas-Gracia, L.M.; Martín-Ramos, P.; Pérez-Lebeña, E.; Martín-Gil, J. A kinetic study on microwave-assisted conversion of cellulose and lignocellulosic waste into hydroxymethylfurfural/furfural. Bioresour. Technol. 2015, 180, 88–96. [Google Scholar] [CrossRef] [PubMed]








| Catalysts | Yield to HMF (%) | |
|---|---|---|
| Fructose/ChCl | Glucose/ChCl | |
| FeCl3 | 59 | 15 |
| ZnCl2 | 8 | 6 |
| CrCl2 | 40 | 45 |
| CrCl3 | 60 | 31 |
| AlCl3∙6H2O | - | 70 1 |
| Brønsted Acids | |||||
|---|---|---|---|---|---|
| p-TsOH | p-TSA | CO2 | BHC | GO | |
| Yield to HMF (%) | 59 | 91 | 74 | 84 | 76 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jérôme, F.; De Oliveira Vigier, K. Catalytic Conversion of Carbohydrates to Furanic Derivatives in the Presence of Choline Chloride. Catalysts 2017, 7, 218. https://doi.org/10.3390/catal7070218
Jérôme F, De Oliveira Vigier K. Catalytic Conversion of Carbohydrates to Furanic Derivatives in the Presence of Choline Chloride. Catalysts. 2017; 7(7):218. https://doi.org/10.3390/catal7070218
Chicago/Turabian StyleJérôme, François, and Karine De Oliveira Vigier. 2017. "Catalytic Conversion of Carbohydrates to Furanic Derivatives in the Presence of Choline Chloride" Catalysts 7, no. 7: 218. https://doi.org/10.3390/catal7070218