A Reusable Palladium/Cationic 2,2′-Bipyridyl System-Catalyzed Double Mizoroki-Heck Reaction in Water
Abstract
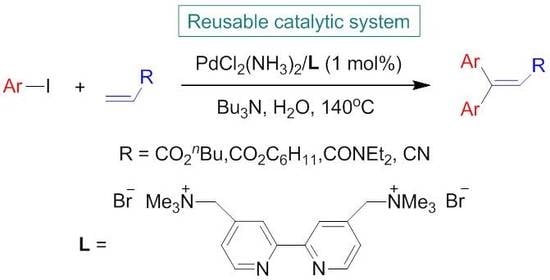
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Typical Procedure for the Double Mizoroki-Heck Reaction
3.3. Typical Procedure for the Reuse of Catalytic Aqueous Solution for the Double Mizoroki-Heck Reaction
3.4. Typical Procedure for the Synthesis of Unsymmetrical β,β-diarylated Alkenes
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gołębiewski, W.M.; Wilkowska, E. Synthesis and biological activity of new diarylalkenes. Polish J. Chem. 2000, 74, 759–766. [Google Scholar]
- Gołębiewski, W.M.; Keyes, R.F.; Cushman, M. Exploration of the effects of linker chain modifications on anti-HIV activities in a series of cosalane analogues. Bioorg. Med. Chem. 1996, 4, 1637–1648. [Google Scholar] [CrossRef]
- Cushman, M.; Gołębiewski, W.M.; Graham, L.; Turpin, J.A.; Rice, W.G.; Fliakas-Boltz, V.; Buckheit, R.W., Jr. Synthesis and biological evaluation of certain alkenyldiarylmethanes as anti-HIV-1 agents which act as non-nucleoside reverse transcriptase inhibitors. J. Med. Chem. 1996, 39, 3217–3227. [Google Scholar] [CrossRef] [PubMed]
- Keyes, R.F.; Gołębiewski, W.M.; Cushman, M. Correlation of anti-HIV potency with lipophilicity in a series of cosalane analogs having normal alkenyl and phosphodiester chains as cholestane replacements. J. Med. Chem. 1996, 39, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M.; Gołębiewski, W.M.; Pommier, Y.; Mazumder, A.; Reymen, D.; De Clercq, E.; Graham, L.; Rice, W.G. Cosalane analogs with enhanced potencies as inhibitors of HIV-1 protease and integrase. J. Med. Chem. 1995, 38, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M.; Gołębiewski, W.M.; McMahon, J.B.; Buckheit, R.W., Jr.; Clanton, D.J.; Weislow, O.; Haugwitz, R.D.; Bader, J.P.; Graham, L.; Rice, W.G. Design, synthesis, and biological evaluation of cosalane, a novel anti-HIV agent which inhibits multiple features of virus reproduction. J. Med. Chem. 1994, 37, 3040–3050. [Google Scholar] [CrossRef] [PubMed]
- Gołębiewski, W.M.; Bader, J.P.; Cushman, M. Design and synthesis of cosalane, a novel anti-HIV agent. Bioorg. Med. Chem. Lett. 1993, 3, 1739–1742. [Google Scholar] [CrossRef]
- Colwell, W.T.; Lange, J.H.; Henry, D.W. 5,5-Diarylpenta-2,4-dienoic acid amides as potential antimalarial agents. J. Med. Chem. 1968, 11, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Ruchelman, A.L.; Man, H.-W.; Chen, R.; Liu, W.; Lu, L.; Cedzik, D.; Zhang, L.; Leisten, J.; Collette, A.; Narla, R.K.; et al. 1,1-Diarylalkenes as anticancer agents: Dual inhibitors of tubulin polymerization and phosphodiesterase 4. Bioorg. Med. Chem. 2011, 19, 6356–6374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-H.; Wu, L.; Raymon, H.K.; Chen, R.S.; Corral, L.; Shirley, M.A.; Narla, R.K.; Gamez, J.; Muller, G.W.; Stirling, D.I.; et al. The synthetic compound CC-5079 is a potent inhibitor of tubulin polymerization and tumor necrosis factor-production with antitumor activity. Cancer Res. 2006, 66, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Xu, H.; Zhang, N.; Chen, W. Triazole-functionalized N-heterocyclic carbene complexes of palladium and platinum and efficient aqueous Suzuki-Miyaura coupling reaction. Chem. Asian J. 2010, 5, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Kabalka, G.W.; Dong, G.; Venkataiah, B. Synthesis of conjugated enediynes via palladium catalyzed cross-coupling reactions of potassium alkynyltrifluoroborates. Tetrahedron Lett. 2005, 46, 763–765. [Google Scholar] [CrossRef]
- Bauer, A.; Miller, M.W.; Vice, S.F.; McCombie, S.W. Suzuki arylation of 1,1-dibromo-1-alkenes: Synthesis of tetra-substituted alkenes. Synlett 2001, 2001, 254–256. [Google Scholar]
- Shen, W. A modified Suzuki reaction of 1,1-dibromo-1-alkenes. Synlett 2000, 2000, 737–739. [Google Scholar]
- Itami, K.; Mineno, M.; Muraoka, N.; Yoshida, J. Sequential assembly strategy for tetrasubstituted olefin synthesis using vinyl 2-pyrimidyl sulfide as a platform. J. Am. Chem. Soc. 2004, 126, 11778–11779. [Google Scholar] [CrossRef] [PubMed]
- Itami, K.; Tonogaki, K.; Ohashi, Y.; Yoshida, J. Rapid construction of multisubstituted olefin structures using vinylboronate ester platform leading to highly fluorescent materials. Org. Lett. 2004, 6, 4093–4096. [Google Scholar] [CrossRef] [PubMed]
- Svennebring, A.; Nilsson, P.; Larhed, M. Microwave-promoted and chelation-controlled double arylations of terminal olefinic carbon of vinyl ethers. J. Org. Chem. 2004, 69, 3345–3349. [Google Scholar] [CrossRef] [PubMed]
- Itami, K.; Nokami, T.; Ishimura, Y.; Mitsudo, K.; Kamei, T.; Yoshida, J. Diversity-oriented synthesis of multisubstituted olefins through the sequential integration of palladium-catalyzed cross-coupling reactions. 2-Pyridyldimethyl(vinyl)silane as a versatile platform for olefin synthesis. J. Am. Chem. Soc. 2001, 123, 11577–11585. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Maňas, M.; Pérez, M.; Pleixats, R. Stereospecific preparation of ethyl (E) and (Z)-3-aryl-e-phenylpropenoates by Heck reaction. Tetrahedron Lett. 1996, 37, 7449–7452. [Google Scholar] [CrossRef]
- Sugihara, T.; Takebayashi, M.; Kaneko, C. Effects of high pressure on the Heck reaction. Is it possible to control dehydropalladation of alkylpalladium intermediates having-hydrogens? Tetrahedron Lett. 1995, 36, 5547–5550. [Google Scholar] [CrossRef]
- Cortese, N.A.; Ziegler, C.B., Jr.; Hrnjez, B.J.; Heck, R.F. Palladium-catalyzed synthesis of 2-quinolone derivatives from 2-iodoanilines. J. Org. Chem. 1978, 43, 2952–2958. [Google Scholar] [CrossRef]
- Calò, V.; Nacci, A.; Monopoli, A.; Lopez, L.; di Cosmo, A. Heck reaction of β-substituted acrylates in ionic liquids catalyzed by a Pd-benzothiazole carbene complex. Tetrahedron 2001, 57, 6071–6077. [Google Scholar] [CrossRef]
- Smith, M.R.; Kim, J.Y.; Ciufolini, M.A. Pd—Arylurea complexes for the Heck arylation of crotonic and cinnamic substrates. Tetrahedron Lett. 2013, 54, 2042–2045. [Google Scholar] [CrossRef]
- Smith, M.R.; Jang, Y.J.; Kim, Y.J.; Ciufolini, M.A. Selective reactivity of electron-rich aryl iodides in the Heck arylation of disubstituted alkenes catalyzed by palladium-arylurea complexes. Tetrahedron 2013, 69, 10139–10151. [Google Scholar] [CrossRef]
- Xu, D.; Lu, C.; Chen, W. Palladium-catalyzed double arylations of terminal olefins in acetic acid. Tetrahedron 2012, 68, 1466–1474. [Google Scholar] [CrossRef]
- Zhu, Z.-Q.; He, J.-S.; Wang, H.-J.; Huang, Z.-Z. Domino reactions containing different types of Heck reactions for selective 3,3- and 1,3-diarylations of propenol with aryl halides by triple catalysis. J. Org. Chem. 2015, 80, 9354–9359. [Google Scholar] [CrossRef] [PubMed]
- Choudary, B.M.; Sarma, R.M.; Rao, K.K. A highly active and stereoselective montmorillonite catalyst for arylation of conjugated alkenes. Tetrahedron 1992, 48, 719–726. [Google Scholar] [CrossRef]
- Shil, A.K.; Das, P. Polystyrene resin supported palladium(0) (Pd@PR) nanocomposite catalyzed synthesis of β-aryl and β,β-diaryl unsaturated scaffolds following tandem approaches. RSC Adv. 2015, 5, 24859–24863. [Google Scholar] [CrossRef]
- Mastrorilli, P.; Dell’Anna, M.M.; Rizzuti, A.; Mali, M.; Zapparoli, M.; Leonelli, C. Resin-immobilized palladium nanoparticle catalysts for organic reactions in aqueous media: Morphological aspects. Molecules 2015, 20, 18661–18684. [Google Scholar] [CrossRef] [PubMed]
- Mastrorilli, P.; Monopoli, A.; Dell’Anna, M.M.; Latronico, A.; Cotugno, P.; Nacci, A. Ionic liquids in palladium-catalyzed cross-coupling reactions. Top. Organomet. Chem. 2015, 51, 237–286. [Google Scholar]
- Cotugno, P.; Casiello, M.; Nacci, A.; Mastrorilli, P.; Dell’Anna, M.M.; Monopoli, A. Suzuki coupling of iodo and bromoarenes catalyzed by chitosan-supported Pd-nanoparticles in ionic liquids. J. Organomet. Chem. 2014, 752, 1–5. [Google Scholar] [CrossRef]
- Dell’Anna, M.M.; Mali, M.; Mastrorilli, P.; Rizzuti, A.; Ponzoni, C.; Leonelli, C. Suzuki—Miyaura coupling under air in water promoted by polymer supported palladium nanoparticles. J. Mol. Catal. A Chem. 2013, 366, 186–194. [Google Scholar] [CrossRef]
- Park, S.B.; Alper, H. Highly efficient, recyclable Pd(II) catalysts with bisimidazole ligands for the Heck reaction in ionic liquids. Org. Lett. 2003, 5, 3209–3212. [Google Scholar] [CrossRef] [PubMed]
- Delample, M.; Villandier, N.; Douliez, J.-P.; Camy, S.; Condoret, J.-S.; Pouilloux, Y.; Barrault, J.; Jérôme, F. Glycerol as a cheap, safe and sustainable solvent for the catalytic and regioselective-diarylation of acrylates over palladium nanoparticles. Green Chem. 2010, 12, 804–808. [Google Scholar] [CrossRef]
- Levin, E.; Ivry, E.; Diesendruck, C.E.; Lemcoff, N.G. Water in N-heterocyclic carbene-assisted catalysis. Chem. Rev. 2015, 115, 4607–4692. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dixneuf, P.H. sp2 C–H bond activation in water and catalytic cross-coupling reactions. Chem. Soc. Rev. 2013, 42, 5744–5767. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.-O.; Li, C.-J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.N.; Coyne, A.G. Water: Nature’s reaction enforcer—Comparative effects for organic synthesis “in-water” and “on-water”. Chem. Rev. 2010, 110, 6302–6337. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, K.H. Hydrophilic ligands and their application in aqueous-phase metal-catalyzed reactions. Chem. Rev. 2009, 109, 643–710. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.T. Solvents from nature. Green Chem. 2008, 10, 1024–1028. [Google Scholar] [CrossRef]
- Li, C.-J.; Trost, B.M. Green chemistry for chemical synthesis. Proc. Natl. Acad. Sci. USA 2008, 105, 13197–13202. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiao, J. Toward green catalytic synthesis—Transition metal-catalyzed reactions in non-conventional media. J. Mol. Catal. A Chem. 2007, 270, 1–43. [Google Scholar] [CrossRef]
- Dallinger, D.; Kappe, C.O. Microwave-assisted synthesis in water as solvent. Chem. Rev. 2007, 107, 2563–2591. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.T.; Anastas, P.T. Introduction: Green chemistry. Chem. Rev. 2007, 107, 2167–2168. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-J.; Chen, L. Organic chemistry in water. Chem. Soc. Rev. 2006, 35, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-J. Organic reactions in aqueous media with a focus on carbon—Carbon bond formations: A decade update. Chem. Rev. 2005, 105, 3095–3166. [Google Scholar] [CrossRef] [PubMed]
- Leadbeater, N.E. Fast, easy, clean chemistry by using water as a solvent and microwave heating: The Suzuki coupling as an illustration. Chem. Commun. 2005, 2881–2902. [Google Scholar] [CrossRef] [PubMed]
- Botella, L.; Nájera, C. Controlled mono and double Heck reactions in water catalyzed by an oxime-derived palladacycle. Tetrahedron Lett. 2004, 45, 1833–1836. [Google Scholar] [CrossRef]
- Botella, L.; Nájera, C. Mono- and β,β-double-Heck reactions of α,β-unsaturated carbonyl compounds in aqueous media. J. Org. Chem. 2005, 70, 4360–4369. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Chen, J.-R.; Tsai, F.-Y. Palladium(II)/cationic 2,2′-bipyridyl system as a highly efficient and reusable catalyst for the Mizoroki-Heck reaction in water. Molecules 2010, 15, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Y.; Chen, S.-N.; Tsai, F.-Y. Recyclable and highly active cationic 2,2′-bipyridyl palladium(II) catalyst for Suzuki cross-coupling reaction in water. Tetrahedron Lett. 2006, 47, 9267–9270. [Google Scholar] [CrossRef]
- Chen, S.-N.; Wu, W.-Y.; Tsai, F.-Y. Hiyama reaction of aryl bromides with arylsiloxanes catalyzed by a reusable palladium(II)/cationic bipyridyl system in water. Tetrahedron 2008, 64, 8164–8168. [Google Scholar] [CrossRef]
- Suzuki, T.; Nagae, O.; Kato, Y.; Nakagawa, H.; Fukuhara, K.; Miyata, N. Photoinduced nitric oxide release from nitrobenzene derivatives. J. Am. Chem. Soc. 2005, 127, 11720–11726. [Google Scholar] [CrossRef] [PubMed]
- Sirasani, G.; Tong, L.; Balskus, E.P. A biocompatible alkene hydrogenation merges organic synthesis with microbial metabolism. Angew. Chem. Int. Ed. 2014, 53, 7785–7788. [Google Scholar] [CrossRef] [PubMed]

| Entry | Aryl Iodide | Product | Yield (%) b |
|---|---|---|---|
| 1 |  1a 1a |  3a 3a | 95 c 90 d 84 e |
| 2 |  1b 1b |  3b 3b | 86 c 79 d 72 e |
| 3 |  1c 1c |  3c 3c | 68 c 62 d 57 e |
| 4 |  1d 1d |  3d 3d | 97 c 92 d 78 e |
| 5 |  1e 1e |  3e 3e | 95 c 85 d 73 e |
| 6 |  1f 1f |  3f 3f | 93 c 91 d 81 e |
| 7 |  1g 1g |  3g 3g | 92 c 91 d 90 e |
| 8 |  1h 1h |  3h 3h | 60 c 59 d 52 e |
| 9 |  1i 1i |  3i 3i | 68 c 66 d 65 e |
| Entry | Aryl Iodide | Product | Yield (%) b |
|---|---|---|---|
| 1 |  1a 1a |  4a 4a | 98 c 95 d 91 e |
| 2 |  1b 1b |  4b 4b | 78 c 77 d 66 e |
| 3 |  1c 1c |  4c 4c | 72 c 61 d 61 e |
| 4 |  1d 1d |  4d 4d | 99 c 97 d 95 e |
| 5 |  1e 1e |  4e 4e | 96 c 91 d 90 e |
| 6 |  1f 1f |  4f 4f | 90 c 83 d 76 e |
| 7 |  1g 1g |  4g 4g | 99 c 93 d 87 e |
| 8 |  1h 1h |  4h 4h | 47 c 40 d 34 e |
| Entry | Aryl Iodide | Product | Yield (%) b |
|---|---|---|---|
| 1 c |  1a 1a |  5a 5a | 50 e |
| 2 d | 1a | 5a | 60 e |
| 3 | 1a | 5a | 79 e 69 f 63 g |
| 4 |  1b 1b |  5b 5b | 71 e 66 f 57 g |
| 5 |  1c 1c |  5c 5c | 43 e 38 f 32 g |
| 6 |  1d 1d |  5d 5d | 80 e 79 f 72 g |
| 7 |  1e 1e |  5e 5e | 91 e 90 f 82 g |
| 8 |  1f 1f |  5f 5f | 80 e 76 f 68 g |
| 9 |  1g 1g |  5g 5g | 90 e 83 f 77 g |
| 10 |  1h 1h |  5h 5h | 68 e 54 f 47 g |
| Entry | Aryl Iodide | Product | Yield (%) b |
|---|---|---|---|
| 1 |  1a 1a |  6a 6a | 97 c 92 d 83 e |
| 2 |  1b 1b |  6b 6b | 74 c 72 d 64 e |
| 3 |  1c 1c |  6c 6c | 58 c 52 d 47 e |
| 4 |  1d 1d |  6d 6d | 93 c 92 d 88 e |
| 5 |  1e 1e |  6e 6e | 98 c 97 d 94 e |
| 6 |  1f 1f |  6f 6f | 96 c 95 d 93 e |
| 7 |  1g 1g |  6g 6g | 96 c 93 d 83 e |
| 8 |  1h 1h |  6h 6h | 89 c 86 d 81 e |
| 9 f |  1j 1j |  6j 6j | 88 c 76 d 68 e |
| 10 f |  1k 1k |  6k 6k | 92 c 82 d 70 e |
| Entry | Aryl Iodide | Alkene | Product | Yield (%) b | E/Z c |
|---|---|---|---|---|---|
| 1 |  1e 1e |  7a 7a |  8ae 8ae | 94 | 2.3/1 |
| 2 |  1h 1h | 7a |  8ah 8ah | 74 | 1.0/1 |
| 3 | 1e |  7b 7b |  8be 8be | 88 | 2.4/1 |
| 4 | 1h | 7b |  8bh 8bh | 80 | 1.0/1 |
| 5 d | 1e |  7c 7c |  8ce 8ce | 98 | 12.4/1 |
| 6 d | 1h | 7c |  8ch 8ch | 91 | 11.1/1 |
| 7 | 1e |  7d 7d |  8de 8de | 97 | 6.5/1 |
| 8 | 1h | 7d |  8dh 8dh | 91 | 4.7/1 |
| 9 |  1j 1j |  7e 7e |  8ej 8ej | 90 | 1/5.2 |
| 10 |  1k 1k |  7f 7f | 8ej | 97 | 4.3/1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Wu, C.-C.; Liao, W.-T.; Liu, L.-J.; Tsai, F.-Y. A Reusable Palladium/Cationic 2,2′-Bipyridyl System-Catalyzed Double Mizoroki-Heck Reaction in Water. Catalysts 2017, 7, 177. https://doi.org/10.3390/catal7060177
Chen Y-C, Wu C-C, Liao W-T, Liu L-J, Tsai F-Y. A Reusable Palladium/Cationic 2,2′-Bipyridyl System-Catalyzed Double Mizoroki-Heck Reaction in Water. Catalysts. 2017; 7(6):177. https://doi.org/10.3390/catal7060177
Chicago/Turabian StyleChen, Yu-Chi, Chien-Chi Wu, Wei-Ting Liao, Ling-Jun Liu, and Fu-Yu Tsai. 2017. "A Reusable Palladium/Cationic 2,2′-Bipyridyl System-Catalyzed Double Mizoroki-Heck Reaction in Water" Catalysts 7, no. 6: 177. https://doi.org/10.3390/catal7060177