Solvothermal Crystallization of Ag/AgxO-AgCl Composites: Effect of Different Chloride Sources/Shape-Tailoring Agents
Abstract
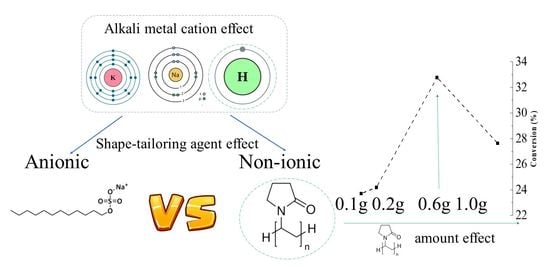
:1. Introduction
- the presence of LSPR facilitates the utilization of visible light by the plasmonic effect of the Ag nanoparticles that gives rise to an electronic absorption signal in this spectral domain [21];
- the Schottky barrier effect occurs when an electron is quickly moving from the semiconductor to the noble metal, often leading to the favorable change of the Fermi level in terms of photocatalytic efficiency [22].
- the solvothermal synthesis of AgCl microcrystals at 160 °C;
- the synergic effect of the used chloride sources (HCl, NaCl, and KCl);
- the nature of shape-tailoring agents (non-ionic: PVP and anionic: SDS) on the AgCl microcrystals and their stability;
- the effect of the as-appeared Ag nanoparticles (during the synthesis/photocatalytic degradation of methyl orange (MO)) on the photocatalytic activity and stability of the AgCl microcrystals.
2. Results
2.1. Characterization of AgCl Microcrystals
- the crystallization of AgCl was incomplete, showing a partially crystallized structure with crystal lattice defects, since the “shoulders” follow the original AgCl diffraction peaks.
- other Ag-based structures were formed (the most probable ones are AgO‒32.0° (COD file no. 00-150-9488); Ag2O‒46.2° (COD file no. 00-101-0486); and AgClO2‒32.37° (COD file no. 00-231-0062)).
2.2. Photocatalytic Efficiency of the Samples
2.2.1. AgCl_NØ Sample Series
2.2.2. AgCl_KCl Sample Series
2.2.3. AgCl_NaCl Sample Series
2.2.4. AgCl_HCl Sample Series
2.3. Effect of Polyvinylpyrrolidone (PVP) Amount on the Properties and Photocatalytic Activity of AgCl_HCl Sample Series
2.4. Surface Structure of AgCl Materials
2.5. Structural and Optical Parameters of AgCl after Degradation Processes
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Silver Chloride Particles
3.3. Characterization Methods, Instrumentation, and Assessment of Photocatalytic Efficiencies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Fang, W.; Yu, C.; Zhou, W.; Zhu, L.; Xie, Y. Ag-based semiconductor photocatalysts in environmental purification. Appl. Surf. Sci. 2015, 358, 46–56. [Google Scholar] [CrossRef]
- Xue, W.; Huang, D.; Wen, X.; Chen, S.; Cheng, M.; Deng, R.; Li, B.; Yang, Y.; Liu, X. Silver-based semiconductor Z-scheme photocatalytic systems for environmental purification. J. Hazard. Mater. 2020, 390, 122128. [Google Scholar] [CrossRef] [PubMed]
- Pargar, F.; Koleva, D.A.; van Breugel, K. Determination of Chloride Content in Cementitious Materials: From Fundamental Aspects to Application of Ag/AgCl Chloride Sensors. Sensors 2017, 17, 2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadovnikov, S.; Kozlova, E.; Gerasimov, E.; Rempel, A. Photocatalytic hydrogen evolution from aqueous solutions on nanostructured Ag2S and Ag2S/Ag. Catal. Commun. 2017, 100, 178–182. [Google Scholar] [CrossRef]
- Xue, J.; Liu, J.; Liu, Y.; Li, H.; Wang, Y.; Sun, D.; Wang, W.; Huang, L.; Tang, J. Recent advances in synthetic methods and applications of Ag2S-based heterostructure photocatalysts. J. Mater. Chem. C 2019, 7, 3988–4003. [Google Scholar] [CrossRef]
- Buckley, J.J.; Lee, A.F.; Olivi, L.; Wilson, K. Hydroxyapatite supported antibacterial Ag3PO4 nanoparticles. J. Mater. Chem. 2010, 20, 8056–8063. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, S.; Yu, H.; Yu, J.; Liu, S. Ag2O as a new visible-light photocatalyst: Self-stability and high photocatalytic activity. Chem. Eur. J. 2011, 17, 7777–7780. [Google Scholar] [CrossRef]
- Daupor, H.; Wongnawa, S. Flower-like Ag/AgCl microcrystals: Synthesis and photocatalytic activity. Mater. Chem. Phys. 2015, 159, 71–82. [Google Scholar] [CrossRef]
- Tang, H.; Wang, Y.; Zhang, D.; Wu, K.; Huang, H. Shape-controllable synthesis and morphology-dependent photocatalytic properties of AgBr photocatalysts. J. Mater. Sci. Mater. Electron. 2016, 27, 6955–6963. [Google Scholar] [CrossRef]
- An, C.; Liu, J.; Wang, S.; Zhang, J.; Wang, Z.; Long, R.; Sun, Y. Concaving AgI sub-microparticles for enhanced photocatalysis. Nano Energy 2014, 9, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Dai, Y.; Wang, X. Methods and mechanism for improvement of photocatalytic activity and stability of Ag3PO4: A review. J. Alloys Compd. 2015, 649, 910–932. [Google Scholar] [CrossRef]
- Dong, H.; Chen, G.; Sun, J.; Li, C.; Yu, Y.; Chen, D. A novel high-efficiency visible-light sensitive Ag2CO3 photocatalyst with universal photodegradation performances: Simple synthesis, reaction mechanism and first-principles study. Appl. Catal. B Environ. 2013, 134–135, 46–54. [Google Scholar] [CrossRef]
- Dai, G.; Yu, J.; Liu, G. A New Approach for Photocorrosion Inhibition of Ag2CO3 Photocatalyst with Highly Visible-Light-Responsive Reactivity. J. Phys. Chem. C 2012, 116, 15519–15524. [Google Scholar] [CrossRef]
- Tian, J.; Liu, R.; Wang, G.; Xu, Y.; Wang, X.; Yu, H. Dependence of metallic Ag on the photocatalytic activity and photoinduced stability of Ag/AgCl photocatalyst. Appl. Surf. Sci. 2014, 319, 324–331. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.H.; Maternaghan, T.J. Photographic Materials. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Sophocleous, M.; Atkinson, J.K. A review of screen-printed silver/silver chloride (Ag/AgCl) reference electrodes potentially suitable for environmental potentiometric sensors. Sens. Actuators A Phys. 2017, 267, 106–120. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Ge, L.; Chen, C.; Li, Y.; Zhao, Z.; Xiao, X.; Li, Z.; Zhang, J. Site-selected synthesis of novel Ag@AgCl nanoframes with efficient visible light induced photocatalytic activity. J. Mater. Chem. A 2014, 2, 12594–12600. [Google Scholar] [CrossRef]
- Han, L.; Wang, P.; Zhu, C.; Zhai, Y.; Dong, S. Facile solvothermal synthesis of cube-like Ag@AgCl: A highly efficient visible light photocatalyst. Nanoscale 2011, 3, 2931–2935. [Google Scholar] [CrossRef] [PubMed]
- Sanzone, G.; Zimbone, M.; Cacciato, G.; Ruffino, F.; Carles, R.; Privitera, V.; Grimaldi, M. Ag/TiO2 nanocomposite for visible light-driven photocatalysis. Superlattices Microstruct. 2018, 123, 394–402. [Google Scholar] [CrossRef]
- Ziashahabi, A.; Prato, M.; Dang, Z.; Poursalehi, R.; Naseri, N. The effect of silver oxidation on the photocatalytic activity of Ag/ZnO hybrid plasmonic/metal-oxide nanostructures under visible light and in the dark. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Ye, M.; Iocozzia, J.; Lin, C.; Lin, Z. Plasmon-Mediated Solar Energy Conversion via Photocatalysis in Noble Metal/Semiconductor Composites. Adv. Sci. 2016, 3, 1600024. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chen, Y.L.; Liu, R.-S.; Tsai, D.P. Plasmonic photocatalysis. Rep. Prog. Phys. 2013, 76, 046401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Deng, D.; Tian, Q. One pot controllable synthesis of AgCl nanocrystals with different morphology and their photocatalytic activity. Powder Technol. 2017, 308, 206–213. [Google Scholar] [CrossRef]
- Bi, Y.; Ye, J. In situ oxidation synthesis of Ag/AgCl core–shell nanowires and their photocatalytic properties. Chem. Commun. 2009, 10, 6551–6553. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, P.; Ma, W.; Lei, B.; Liu, M. Template-Free Synthesis of Cube-like Ag/AgCl Nanostructures via a Direct-Precipitation Protocol: Highly Efficient Sunlight-Driven Plasmonic Photocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 6386–6392. [Google Scholar] [CrossRef]
- Wu, S.; Shen, X.; Ji, Z.; Zhu, G.; Zhou, H.; Zang, H.; Yu, T.; Chen, C.; Song, C.; Feng, L.; et al. Morphological syntheses and photocatalytic properties of well-defined sub-100 nm Ag/AgCl nanocrystals by a facile solution approach. J. Alloys Compd. 2017, 693, 132–140. [Google Scholar] [CrossRef]
- Mohandes, F.; Salavati-Niasari, M. Application of a new coordination compound for the preparation of AgI nanoparticles. Mater. Res. Bull. 2013, 48, 3773–3782. [Google Scholar] [CrossRef]
- Elsaeedy, H. A low temperature synthesis of Ag2S nanostructures and their structural, morphological, optical, dielectric and electrical studies: An effect of SDS surfactant concentration. Mater. Sci. Semicond. Process. 2019, 93, 360–365. [Google Scholar] [CrossRef]
- Xu, Z.; Han, L.; Hu, P.; Dong, S. Facile synthesis of small Ag@AgCl nanoparticles via a vapor diffusion strategy and their highly efficient visible-light-driven photocatalytic performance. Catal. Sci. Technol. 2014, 4, 3615–3619. [Google Scholar] [CrossRef]
- Cui, L.; Peng, Q.; Jiao, T.; Zhang, Q.; Zhou, J. Facile Preparation of Silver Halide Nanoparticles as Visible Light Photocatalysts. Nanomater. Nanotechnol. 2015, 5, 20. [Google Scholar] [CrossRef]
- Tang, Y.; Jiang, Z.; Xing, G.; Li, A.; Kanhere, P.D.; Zhang, Y.; Sum, T.C.; Li, S.; Chen, X.; Dong, Z.; et al. Efficient Ag@AgCl Cubic Cage Photocatalysts Profit from Ultrafast Plasmon-Induced Electron Transfer Processes. Adv. Funct. Mater. 2013, 23, 2932–2940. [Google Scholar] [CrossRef]
- Gatemala, H.; Thammacharoen, C.; Ekgasit, S. 3D AgCl microstructures selectively fabricated via Cl−-induced precipitation from [Ag(NH3)2]+. CrystEngComm 2014, 16, 6688–6696. [Google Scholar] [CrossRef]
- Mostafa, M.; Ramadan, H.; El-Amir, M. Sorption and desorption studies of radioiodine onto silver chloride via batch equilibration with its aqueous media. J. Environ. Radioact. 2015, 150, 9–19. [Google Scholar] [CrossRef]
- Ao, Y.; Bao, J.; Wang, P.; Wang, C. A novel heterostructured plasmonic photocatalyst with high photocatalytic activity: Ag@AgCl nanoparticles modified titanium phosphate nanoplates. J. Alloys Compd. 2017, 698, 410–419. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, I.; Ham, J.; Gim, S.; Lee, J.-L. Simple and scalable growth of AgCl nanorods by plasma-assisted strain relaxation on flexible polymer substrates. Nat. Commun. 2017, 8, 15650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Assis, M.; Filho, F.C.G.; Pimentel, D.S.; Robeldo, T.; Gouveia, A.F.; Castro, T.F.D.; Fukushima, H.C.S.; de Foggi, C.C.; da Costa, J.P.C.; Borra, R.C.; et al. Ag Nanoparticles/AgX (X = Cl, Br and I) Composites with Enhanced Photocatalytic Activity and Low Toxicological Effects. Chemistry 2020, 5, 4655–4673. [Google Scholar] [CrossRef]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A Study of the Surface Plasmon Resonance of Silver Nanoparticles by the Discrete Dipole Approximation Method: Effect of Shape, Size, Structure, and Assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Yang, S.; Xu, D.; Chen, B.; Luo, B.; Shi, W. In-situ synthesis of a plasmonic Ag/AgCl/Ag2O heterostructures for degradation of ciprofloxacin. Appl. Catal. B Environ. 2017, 204, 602–610. [Google Scholar] [CrossRef]
- Shume, W.M.; Murthy, H.C.A.; Zereffa, E.A. A Review on Synthesis and Characterization of Ag2O Nanoparticles for Photocatalytic Applications. J. Chem. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Baia, L.; Muresan, D.; Baia, M.; Popp, J.; Simon, S. Structural properties of silver nanoclusters–phosphate glass composites. Vib. Spectrosc. 2007, 43, 313–318. [Google Scholar] [CrossRef]
- Shen, X.S.; Wang, G.Z.; Hong, X.; Zhu, W. Nanospheres of silver nanoparticles: Agglomeration, surface morphology control and application as SERS substrates. Phys. Chem. Chem. Phys. 2009, 11, 7450–7454. [Google Scholar] [CrossRef]
- Tóth, Z.-R.; Hernadi, K.; Baia, L.; Kovács, G.; Pap, Z. Controlled formation of Ag-AgxO nanoparticles on the surface of commercial TiO2 based composites for enhanced photocatalytic degradation of oxalic acid and phenol. Catal. Today 2020, in press. [Google Scholar] [CrossRef]
- Tóth, Z.-R.; Kovács, G.; Hernádi, K.; Baia, L.; Pap, Z. The investigation of the photocatalytic efficiency of spherical gold nanocages/TiO2 and silver nanospheres/TiO2 composites. Sep. Purif. Technol. 2017, 183, 216–225. [Google Scholar] [CrossRef]
- Yinghua, L.; Huan, W.; Li, L.; Wenquan, C. Facile Synthesis of Ag@AgCl Plasmonic Photocatalyst and Its Photocatalytic Degradation under Visible Light. Rare Met. Mater. Eng. 2015, 44, 1088–1093. [Google Scholar] [CrossRef]
- Kaushik, V.K. XPS core level spectra and Auger parameters for some silver compounds. J. Electron. Spectrosc. Relat. Phenom. 1991, 56, 273–277. [Google Scholar] [CrossRef]
- Hammond, J.S.; Gaarenstroom, S.W.; Winograd, N. X-ray photoelectron spectroscopic studies of cadmium- and silver-oxygen surfaces. Anal. Chem. 1975, 47, 2193–2199. [Google Scholar] [CrossRef]
- Weightman, P.; Andrews, P.T. The influence of the 4d bandwidth on the M4,5N4,5N4,5 Auger spectra of Ag in MgAg and AlAg alloys. J. Phys. C Solid State Phys. 1980, 13, 3529–3546. [Google Scholar] [CrossRef]
- Weng, B.; Qi, M.-Y.; Han, C.; Tang, Z.-R.; Xu, Y.-J. Photocorrosion Inhibition of Semiconductor-Based Photocatalysts: Basic Principle, Current Development, and Future Perspective. ACS Catal. 2019, 9, 4642–4687. [Google Scholar] [CrossRef]
- Sun, L.; Liu, C.; Li, J.; Zhou, Y.; Wang, H.; Huo, P.; Ma, C.; Yan, Y. Fast electron transfer and enhanced visible light photocatalytic activity by using poly-o-phenylenediamine modified AgCl/g-C3N4 nanosheets. Chin. J. Catal. 2019, 40, 80–94. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, Z.-R.; Maity, S.K.; Gyulavári, T.; Bárdos, E.; Baia, L.; Kovács, G.; Garg, S.; Pap, Z.; Hernadi, K. Solvothermal Crystallization of Ag/AgxO-AgCl Composites: Effect of Different Chloride Sources/Shape-Tailoring Agents. Catalysts 2021, 11, 379. https://doi.org/10.3390/catal11030379
Tóth Z-R, Maity SK, Gyulavári T, Bárdos E, Baia L, Kovács G, Garg S, Pap Z, Hernadi K. Solvothermal Crystallization of Ag/AgxO-AgCl Composites: Effect of Different Chloride Sources/Shape-Tailoring Agents. Catalysts. 2021; 11(3):379. https://doi.org/10.3390/catal11030379
Chicago/Turabian StyleTóth, Zsejke-Réka, Saurav Kumar Maity, Tamás Gyulavári, Enikő Bárdos, Lucian Baia, Gábor Kovács, Seema Garg, Zsolt Pap, and Klara Hernadi. 2021. "Solvothermal Crystallization of Ag/AgxO-AgCl Composites: Effect of Different Chloride Sources/Shape-Tailoring Agents" Catalysts 11, no. 3: 379. https://doi.org/10.3390/catal11030379