1. Introduction
The estrogenic hormone 17α-ethynylestradiol (EE2) belongs to a group of pollutants termed estrogenic endocrine-disrupting chemicals (E-EDC), which are chemical substances in the environment that have a negative impact on the endocrine systems of animals and also humans [
1]. E-EDCs enter the environment in the form of waste products as a result of elevated production and consumption of a number of drugs, e.g., hormonal contraceptives or supportive substances [
2], and already constitute a toxicological reproductive risk at very low concentrations (ng/L). These substances can, in fact, simulate the behavior of endogenic estrogenic hormones, which control a number of physiological processes, including sperm maturation and preparation for fertilization, binding to their receptors [
2,
3,
4] (estrogen receptors (ERs), nuclear receptors (nER), and membrane (mER) and cytoplasmic (cER) receptors) [
5,
6].
EE2 is a synthetic hormone used in hormonal contraception and is resistant to decomposition in the liver [
7,
8]. After ingestion, it is excreted in the urine, most often as a conjugate (glucuronate or sulphate), and thus enters waste waters, and, after deconjugation, can act as an E-EDC in the environment [
9]. Its subsequent binding to an estrogen receptor can inhibit the secretion of the gonadotropin-releasing hormone and subsequently the secretion of the luteinizing hormone, leading to a reduction in the function or activity of the testes and directly affecting spermatogenesis, i.e., the process of formation and development of sperm, resulting in a male sex cell [
10,
11]. The sperm could then be morphologically mature, but not capable of fertilizing an egg [
12].
Under normal circumstances, following ejaculation into the female reproductive system, sperm undergo complex biochemical and physiological changes, summarily termed capacitation [
13]. Only capacitated sperm gain progressive motility (sperm hyperactivation) and are capable of penetrating through the egg envelope (in a process known as the acrosome reaction) and fertilizing the egg [
14]. Capacitation in vivo, which includes membrane rearrangement, cholesterol efflux, activation of specific signal transduction pathways leading to protein tyrosine phosphorylation (TyrP) and cytoskeleton rearrangements [
15,
16,
17], takes place in the uterus and fallopian tubes and is activated by substances excreted from the female genital tract. The capacitation process in vitro can take place under exactly delimited conditions employing incubation of sperm in a capacitation/fertilization medium containing albumin and calcium ions, at a temperature of 37 °C and in an atmosphere with 5% CO
2, which imitates the physiological environment in female genital tracts [
15,
18]. In order to study the effect of the presence of exogenous hormones on sperm capacitation in vitro, it is important to determine how capable the sperm is of binding these hormones during these processes and the dynamics of this process.
Methods of studying the effect of estrogenic hormones on sperm capacitation have been published only sporadically in the literature. Immunochemical methods have been used to study the effect of estrogens on the capacitation of mouse sperm in vivo [
19] and boar sperm in vitro [
20]. It has been found that sperm capacitation in vitro is substantially modulated by the presence of estrogens and that its speed can also be increased [
21,
22,
23]. The effect of estriol on the capacitation of mouse sperm has been studied by HPLC with UV detection, but with low detection sensitivity [
24]. The method was greatly improved by using sensitive HPLC-MS/MS methods for monitoring the action of 17β-estradiol (E2) on the capacitation of mouse sperm in vitro, enabling monitoring of changes in hormone concentration levels during sperm capacitation even at the level of units of μg/L [
25,
26], thus approaching real values of potential exposure. The obtained results were subjected to kinetic analysis, which has been used successfully to study the mechanism of the action of fluorides and aluminum fluoride complexes on the capacitation ability of mouse sperm [
27]. The results of kinetic analysis of concentration changes on the receptors of unbound E2 during time-dependent sperm capacitation have demonstrated that E2 is first adsorbed on the surface of plasmatic membranes and subsequently passes into the cytoplasm, where it forms an unstable adduct with the receptors; the formation of this adduct has an autocatalytic effect [
26]. It has thus been found that the application of kinetic analysis to the experimentally obtained data could be a useful instrument in monitoring sperm responses to the action of external factors and for prediction of kinetically specific mechanisms at a molecular level, including selected signal pathways.
This study focuses on the effect of synthetic EE2 on mouse sperm capacitation and comparison of the EE2 action with E2 [
26]. An HPLC-MS/MS method capable of monitoring the concentration changes in unbound EE2 during time-dependent capacitation of mouse sperm in capacitating medium is developed, and the results obtained are subjected to kinetic analysis.
4. Materials and Methods
4.1. Chemicals, Reagents, and Animals
Acetonitrile (ACN) for LC-MS, Chromasolv (purity ≥ 99.9%), deuterated β-estradiol-16,16,17-d3 (estradiol-d3) (purity 98%), and commercial capacitating M2 culture media for in vitro sperm capacitation and fertilization (M7167) were purchased from Sigma-Aldrich (Steinheim, Germany). Ethanol (purity ≥ 96% p.a.) was obtained from Lachner (Neratovice, Czech Republic). Paraffin oil was provided by Carl Roth (Karlsruhe, Germany). Formic acid (HCOOH) (purity 98% p.a.) and 17α-ethynylestradiol (purity 98%) were obtained from Merck (Darmstadt, Germany). Deionized water (1.85 μS, Milli-Q water purification system Millipore, Burlington, MA, USA) was used in all the experiments.
Laboratory inbred strain of house mouse BALB/c was used for the experiments. The mice were housed in animal facilities of the Institute of Molecular Genetics of Czech Academy of Science, Prague, and food and water were supplied ad libitum. All the animal procedures and all the experimental protocols were approved by the Animal Welfare Committee of the Czech Academy of Sciences (Animal Ethics Number 66866/2015-MZE-17214, 18 December 2015).
4.2. Instrumentation and Chromatographic Conditions
The HPLC equipment (Agilent Technologies, Waldbronn, Germany) consisted of a 1290 Infinity Series LC (a quaternary pump, degasser, thermostatic auto sampler, and column oven). A Triple Quad LC/MS 6460 tandem mass spectrometer (Agilent Technologies, Waldbronn, Germany) with an electrospray ionization interface was used for the detection. The signal was processed and data were handled using MassHunter Workstation Acquisition and MassHunter Qualitative Analysis Software (Agilent Technologies, Waldbronn, Germany).
All the instrumental MS-MS parameters were optimized. ESI (+) conditions in the MRM mode for EE2 were capillary voltage 4000 V, nebulizer pressure 55 psi, gas temperature 350 °C, and nitrogen flow rate 10 L/min. For EE2 the m/z 279.1 → 133.0 transition (fragmentor voltage 100 V, collision energy 15 V, and parameter dwell 400 ms) and for estradiol-d3 the m/z 258.5 → 158.9 transition (fragmentor voltage 100 V, collision energy 15 V, and parameter dwell 400 ms) were monitored, respectively.
The separation system was based on the publication of Bosakova et al. [
26] with a Kinetex EVO column C18 (100 × 3.0 mm, 2.6 μm, Phenomenex, Torrance, CA, USA) and a mobile phase containing a binary mixture of ACN/water with addition of 0.1% HCOOH in both parts at a volume ratio of 50/50 (
v/
v); the flow rate was 0.3 mL/min. The column temperature was held at 21 ± 0.5 °C. The amount of sample injected equaled 7.5 µL. Estradiol-d3, which was used as an internal standard (IS) at a final concentration of 25 μg/L (diluted in capacitating medium) was added to each sample. The retention times of IS and EE2 were 3.1 and 3.7 min, respectively. Because of the complex capacitating medium containing inorganic and organic components, of which especially bovine serum albumin (4.0 g/L) can cause difficulties during the separation and detection processes, the eluate was fed to waste from 0 to 2.5 min and to the MS detector only from 2.5 to 5.0 min.
The linearity of the method was determined from the calibration curve constructed by plotting the ratio of the peak areas of EE2 to that of IS against the analyte concentration. It was statistically analyzed by 1/x weighted linear regression analysis using the least-squares regression method. The obtained data was linear (y = 0.0252x − 0.0298,
R2 = 0.9989) in the whole measured calibration range 1–225 μg/L. Relative standard deviations (
n’ = 5) varied from 1.8–3.3%. The limit of detection (0.88 μg/L) and limit of quantitation (1.21 μg/L) were calculated as the 3.3 × σ/S and 10 × σ/S ratios, respectively, where σ was the baseline noise of the sample and
S was the slope of the regression curve (based on peak heights) constructed from the calibration curve. The accuracy, precision, and repeatability were measured at three EE2 concentration levels (200, 20, and 2 μg/L). Intra-day and inter-day accuracy and precision (each
n’ = 5) were determined by repeatedly assaying samples on the same day and on two consecutive days, respectively. The results are summarized in
Table 3.
4.3. Capacitation of Mouse Sperm In Vitro
Thirty-five millimeter Petri dishes obtained from Thermo Fisher Scientific (Rochester, NY, USA) were used for the capacitation in vitro. An Olympus CX 21 inverted-light microscope and Olympus epifluorescent microscope were supplied by Olympus (Prague, Czech Republic). An NB-203 incubator was purchased from N-BIOTEK (Gyeonggi-do, Korea). A Telstar Bio-IIA incubator and BioTek laminar box from N-BIOTEK (Gyeonggi-do, Korea) were used for the in vitro sperm cultivation.
Firstly, a stock solution of the EE2 standard with a concentration of 200 mg/L was prepared in ethanol, from which working ethanol solutions of EE2 with concentrations of 20 and 2 mg/L were diluted. In the laminar box, 1 μL of the working ethanol solution of EE2 with the appropriate concentration (200, 20, or 2 mg/L) was pipetted, diluted by capacitating medium to a volume of 1 mL in the test tube so that the final test concentration of EE2 in the capacitating medium was obtained (200, 20, or 2 μg/L), and the ethanol content was minimized and remained constant. Subsequently, 100 μL of solution with the appropriate concentration was pipetted into the fertilization Petri dish. The pipetted mixture in the Petri dish was covered with 1 mL of paraffin oil. The prepared Petri dishes were placed in the incubator and tempered for 60 min at a temperature of 37 °C and with 5% CO2 in the air.
The spermatozoa which were recovered from the distal region of the cauda epididymidis were placed in the fertilization Petri dishes with capacitating medium and paraffin oil and then placed for 10 min in the incubator for sperm release. Then, the stock concentration of mouse sperm was adjusted to 5 × 106 sperm/mL. During preparation, the motility of the sperm was controlled under a microscope.
The biological sample was prepared as follows. Following 60 min of tempering, 5 µL of the stock sperm were added to 100 µL of the EE2 sample in capacitating medium (200, 20, or 2 µg/L). For each capacitation time (0–180 min) eight Petri dishes containing 105 µL of sample covered with 1 mL of paraffin oil were prepared. The dishes prepared in this way were incubated again under the same conditions for various time periods (0, 30, 60, 90, 120, 150, and 180 min after adding the sperm), during which sperm capacitation took place. After the individual times, samples (only the solutions with the capacitating medium and without the paraffin oil), were pipetted from all eight Petri dishes into a single micro-test tube, which was centrifuged for 10 min at 12,000 rpm. In this way the sperm were separated from the solution and approximately 600 μL of supernatant was obtained for HPLC-MS/MS analysis of free, sperm-unbound EE2. This sample represented one sampling time during capacitation.
In order to eliminate any systematic errors during sample preparation (e.g., partial evaporation of samples during incubation or differences in collection of the supernatant after centrifugation, etc.), reference samples (blanks) without addition of mouse sperm were prepared simultaneously under the same experimental conditions.
Prior to the actual HPLC-MS/MS analysis, 20 µL of estradiol-d3 (IS) with a concentration of 250 µg/L was added to each biological sample as well as to each blank (180 µL).
The matrix effect was investigated by comparison of the results (EE2/IS peak area ratios) obtained for samples prepared by two different procedures: (i) the sample was prepared by addition of EE2 and IS to the supernatant, which was obtained after the capacitating medium with sperm covered with paraffin oil was tempered, the paraffin oil removed, and the capacitating medium centrifuged; (ii) the sample was prepared by addition of EE2 and IS to the capacitating medium. Each experiment was performed in triplicate for all EE2 concentrations (200, 20, and 2 µg/L). The recovery percentages of the samples with EE2 concentrations of 200, 20, and 2 μg/L were 94.3, 97.1, and 95.8%, respectively.
4.4. Mathematical Model
As was described in
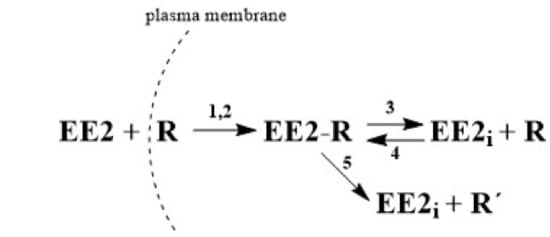
Section 2.2, the term EE2 has been replaced in equations with the symbol
E, which also designates its molar concentration under various experimental conditions.
R designates the receptor and its concentration, symbol
ER stands for the adduct,
Ei is the internal free hormone in the cytoplasmic membrane, and
R’ is the inactive receptor. The formation of the ER adduct following the interaction of hormone
E with receptor
R on the sperm leads to relaxation of the cytoplasmic membrane.
Because the amount of adduct formed is proportional to the difference E(t=0)–E(t) and because the amount of hormone at the end of the reaction equals 0, the degree of relaxation of membrane S can be defined as , meaning S = 1 − ε, where ε is the relative concentration of unbound hormone outside the membrane. Autocatalytic formation of the adduct thus progresses with rate “constant” .
Taking into consideration all the possibilities, this can be described by the following system of kinetic equations, i.e., Equations (1)–(7).
Equation (1) describes the decrease in hormone E by reaction with receptor R by non-autocatalytic (K1) and autocatalytic (K2) reactions, Equation (2) describes the decrease in the receptor through its reaction with the external (K1, K2) and internal hormone (K4) and increase by the adduct decomposition (K3), and Equation (3) describes the increase in the adduct through the non-autocatalytic (K1), autocatalytic (K2), and back (K4) reactions and decrease through the equilibrium (K3) and pseudo-equilibrium (K5) reactions. Equation (4) describes the increase of the internal hormone Ei by the adduct decomposition (K3 and K5) and its decrease by back K4 reaction. Equation (5) describes the formation (K5) of inactive receptor R’.
The balance equation for the hormone (Equation (6)) and the balance equation for the receptor (Equation (7)) are valid, i.e.,
In the kinetic equations, Ei and R can be expressed according to Equations (6) and (7), meaning the set of kinetic equations is reduced to three for variables E, (ER), and R’.
Further, the relative variables are introduced, i.e.,
Equation (8) can also be used to describe the relative concentration of the internal hormone
and the relative concentration of the receptor
in Equations (6) and (7).
Introduction of the relative concentrations and generalized time
yields a set of three differential equations, Equations (10)–(12), i.e.,
where the definitions of
K3’ and
K5’ are
Furthermore, the kinetic scheme employs kinetic products and rate constants in the form of rate equations. The relative values are introduced for the individual variables and the system is solved by numerical integration with simultaneous optimization of the rate constants, including parameter n. Because the sum of the relative concentrations of free hormone B and sperm-bonded hormone α must equal 1, B equals 1–α. Quantity B can be measured and also theoretically calculated from α. For the given kinetic scheme, comparison of the theoretical values with the experimental values makes it possible to decide whether the proposed kinetic scheme is credible.
The equations are solved by the fourth-order Runge-Kutta method with step of
h = 10
−4. The parameters of the theoretical
B(
t) curves, i.e., the rate constants and the molar ratio
n, were optimized by looking for the minima of the absolute values of the difference between the theoretical and experimental
Bt values. The obtained constant values are given in
Table 2 and the shapes of the theoretical curves are depicted in
Figure 3.
5. Conclusions
In this work, an HPLC-MS/MS method was developed and validated for analysis of EE2 concentration in capacitating medium. This method was subsequently used to monitor the concentration changes of the free hormone not bound by mouse sperm during time-dependent capacitation. The experimentally (HPLC-MS/MS) obtained
Bt data were subjected to kinetic analysis and the kinetic model, described by three principal kinetic equations, was designed as follows. Extracellular EE2 can bind to the plasma membrane receptors, forming the adduct in cytoplasm. Once formed, the adduct serves as an autocatalytic agent, acting on the plasma membrane to increase its fluidity. This leads to an increase in the interaction rate between the hormone and the receptor in the cytoplasm. However, the formed adduct is unstable and gradually decomposes. The decomposition can produce the active form of the internal hormone (EE2
i) and active receptor (R) in the cytoplasm, which are both capable of further interactions, enabling a reverse back (equilibrium) reaction. The decomposition can also lead to the deactivation of the receptor (R’) during a unidirectional reaction. Both reactions can take place simultaneously and can be considered to be a pseudo-equilibrium reaction (
Figure 2). The monitoring of EE2 concentration by HPLC/MS-MS in capacitating medium is related to the overall concentration of free unbound hormone, i.e., the original extracellular EE2 together with the internal EE2
i. After centrifugation, both components of the hormone are present in the supernatant, but on the other hand, the hormone bound in the complex with the receptor (adduct: EE2-R) remains in the sediment and its concentration is not measured. Very good agreement of the experimental
Bt values with the theoretically calculated values confirms that the proposed kinetic scheme is credible.
The theoretical shape of the
B(
t) curves obtained for synthetic EE2 mimics behavior of endogenic E2 [
26]; this means that the course has similar autocatalytic character. The differences between EE2 and E2 are in the position of the minimum of
B(
t) curves for the increased initial tested concentration of these two hormones. For EE2, it remains at a constant position of capacitation time and does not depend on the tested concentration. On the other hand, with the increasing E2 concentration, the position of the minimum value shifts to longer times as a result of initial adsorption of E2 on the plasma membrane surface controlled by the Langmuir isotherm. The results suggest that this new analytical–mathematical approach to reproductive biology, such as the study of the mechanism of action of steroid hormones on sperm capacitation, is of a more general nature and can be used in a broader context of endocrine signaling.