Short and Long-Term Mortality Trends for Cancer Patients with Septic Shock Stratified by Cancer Type from 2009 to 2017: A Population-Based Cohort Study
Abstract
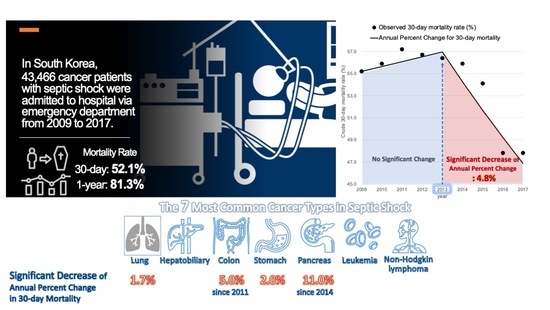
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Study Patients and Data Definitions
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Cancer Patients with Septic Shock Included in this Study
3.2. General Trends in the 30-Day and 1-Year Mortality Outcomes among the Cancer Patients with Septic Shock
3.3. Trends in the 30-Day and 1-Year Mortality among the Cancer Patients with Septic Shock Stratified by Cancer Type
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacobucci, G. Cancer survival in England: Rates improve and variation falls. Br. Med. J. 2019, 365, l1532. [Google Scholar] [CrossRef] [PubMed]
- Sant, M.; Minicozzi, P.; Mounier, M.; Anderson, L.A.; Brenner, H.; Holleczek, B.; Marcos-Gragera, R.; Maynadié, M.; Monnereau, A.; Osca-Gelis, G. Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: Results of EUROCARE-5, a population-based study. Lancet Oncol. 2014, 15, 931–942. [Google Scholar] [CrossRef]
- Coleman, M.; Forman, D.; Bryant, H.; Butler, J.; Rachet, B.; Maringe, C.; Nur, U.; Tracey, E.; Coory, M.; Hatcher, J. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): An analysis of population-based cancer registry data. Lancet 2011, 377, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [Green Version]
- Lemiale, V.; Pons, S.; Mirouse, A.; Tudesq, J.-J.; Hourmant, Y.; Mokart, D.; Pène, F.; Kouatchet, A.; Mayaux, J.; Nyunga, M. Sepsis and Septic Shock in Patients With Malignancies: A Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique Study. Crit. Care Med. 2020, 48, 822–829. [Google Scholar] [CrossRef]
- Cooksley, T.; Rice, T. Emergency oncology: Development, current position and future direction in the USA and UK. Supportive Care Cancer 2017, 25, 3–7. [Google Scholar] [CrossRef]
- Puxty, K.; McLoone, P.; Quasim, T.; Sloan, B.; Kinsella, J.; Morrison, D.S. Risk of critical illness among patients with solid cancers: A population-based observational study. Jama Oncol. 2015, 1, 1078–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schellongowski, P.; Sperr, W.R.; Wohlfarth, P.; Knoebl, P.; Rabitsch, W.; Watzke, H.H.; Staudinger, T. Critically ill patients with cancer: Chances and limitations of intensive care medicine—A narrative review. ESMO Open 2016, 1. [Google Scholar] [CrossRef] [Green Version]
- Taccone, F.S.; Artigas, A.A.; Sprung, C.L.; Moreno, R.; Sakr, Y.; Vincent, J.-L. Characteristics and outcomes of cancer patients in European ICUs. Crit. Care 2009, 13, R15. [Google Scholar] [CrossRef] [Green Version]
- Legrand, M.; Max, A.; Peigne, V.; Mariotte, E.; Canet, E.; Debrumetz, A.; Lemiale, V.; Seguin, A.; Darmon, M.; Schlemmer, B. Survival in neutropenic patients with severe sepsis or septic shock. Crit. Care Med. 2012, 40, 43–49. [Google Scholar] [CrossRef]
- Rosolem, M.M.; Rabello, L.S.; Lisboa, T.; Caruso, P.; Costa, R.T.; Leal, J.V.; Salluh, J.I.; Soares, M. Critically ill patients with cancer and sepsis: Clinical course and prognostic factors. J. Crit. Care 2012, 27, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Ostermann, M.; Ferrando-Vivas, P.; Gore, C.; Power, S.; Harrison, D. Characteristics and outcome of cancer patients admitted to the ICU in England, Wales, and Northern Ireland and national trends between 1997 and 2013. Crit. Care Med. 2017, 45, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.D.; Braun, L.A.; Cooper, L.M.; Johnston, J.; Weiss, R.V.; Qualy, R.L.; Linde-Zwirble, W. Hospitalized cancer patients with severe sepsis: Analysis of incidence, mortality, and associated costs of care. Crit. Care 2004, 8, R291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staudinger, T.; Stoiser, B.; Müllner, M.; Locker, G.J.; Laczika, K.; Knapp, S.; Burgmann, H.; Wilfing, A.; Kofler, J.; Thalhammer, F. Outcome and prognostic factors in critically ill cancer patients admitted to the intensive care unit. Crit. Care Med. 2000, 28, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kang, J.; Kim, M.J.; Ryoo, S.M.; Kang, G.H.; Shin, T.G.; Park, Y.S.; Choi, S.H.; Kwon, W.Y.; Chung, S.P.; et al. Development and validation of the VitaL CLASS score to predict mortality in stage IV solid cancer patients with septic shock in the emergency department: A multi-center, prospective cohort study. BMC Med. 2020, 18, 390. [Google Scholar] [CrossRef]
- Danai, P.A.; Moss, M.; Mannino, D.M.; Martin, G.S. The epidemiology of sepsis in patients with malignancy. Chest 2006, 129, 1432–1440. [Google Scholar] [CrossRef]
- Rivera, D.R.; Gallicchio, L.; Brown, J.; Liu, B.; Kyriacou, D.N.; Shelburne, N. Trends in Adult Cancer-Related Emergency Department Utilization: An Analysis of Data From the Nationwide Emergency Department Sample. Jama Oncol. 2017, 3, e172450. [Google Scholar] [CrossRef]
- Cheol Seong, S.; Kim, Y.-Y.; Khang, Y.-H.; Heon Park, J.; Kang, H.-J.; Lee, H.; Do, C.-H.; Song, J.-S.; Hyon Bang, J.; Ha, S. Data resource profile: The national health information database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Rhee, C.; Murphy, M.V.; Li, L.; Platt, R.; Klompas, M. Comparison of trends in sepsis incidence and coding using administrative claims versus objective clinical data. Clin. Infect. Dis. 2015, 60, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.W.; Oh, C.M.; Choi, H.Y.; Park, J.W.; Cho, H.; Ki, M. Incidence and Overall Survival of Biliary Tract Cancers in South Korea from 2006 to 2015: Using the National Health Information Database. Gut Liv. 2019, 13, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.J.; Oh, I.H.; Yoon, S.J. A comparison of the cancer incidence rates between the national cancer registry and insurance claims data in Korea. Asian Pac. J. Cancer Prev. 2012, 13, 6163–6168. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Won, Y.-J.; Park, Y.R.; Jung, K.-W.; Kong, H.-J.; Lee, E.S. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2017. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2020, 52, 335. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Clin. Epidemiol. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Joinpoint Regression Program. [Computer Software]; Version 4.5.0.1; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute: Bethesda, MD, USA, 2017.
- Kadri, S.S.; Rhee, C.; Strich, J.R.; Morales, M.K.; Hohmann, S.; Menchaca, J.; Suffredini, A.F.; Danner, R.L.; Klompas, M. Estimating ten-year trends in septic shock incidence and mortality in United States academic medical centers using clinical data. Chest 2017, 151, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.-Y.; Cho, S.; Kim, G.H.; Jang, E.J.; Choi, S.; Lee, H.; Ryu, H.G. Incidence and Outcomes of Sepsis in Korea: A Nationwide Cohort Study From 2007 to 2016. Crit. Care Med. 2019, 47, e993–e998. [Google Scholar] [CrossRef]
- Rhee, C.; Murphy, M.V.; Li, L.; Platt, R.; Klompas, M. Improving documentation and coding for acute organ dysfunction biases estimates of changing sepsis severity and burden: A retrospective study. Crit. Care 2015, 19, 338. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.J.; Keller, S.P.; Chan, C.; Glotzbecker, B.E.; Klompas, M.; Baron, R.M.; Rhee, C. Improvements in Sepsis-associated Mortality in Hospitalized Patients with Cancer versus Those without Cancer. A 12-Year Analysis Using Clinical Data. Ann. Am. Thorac. Soc. 2020, 17, 466–473. [Google Scholar] [CrossRef]
- Shin, T.G.; Hwang, S.Y.; Kang, G.H.; Kim, W.Y.; Ryoo, S.M.; Kim, K.; Jo, Y.H.; Chung, S.P.; Joo, Y.S.; Beom, J.H.; et al. Korean Shock Society septic shock registry: A preliminary report. Clin. Exp. Emerg Med. 2017, 4, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Azoulay, E.; Soares, M.; Darmon, M.; Benoit, D.; Pastores, S.; Afessa, B. Intensive care of the cancer patient: Recent achievements and remaining challenges. Ann. Intensive Care 2011, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Jun, J.K.; Choi, K.S.; Lee, H.Y.; Suh, M.; Park, B.; Song, S.H.; Jung, K.W.; Lee, C.W.; Choi, I.J.; Park, E.C.; et al. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology 2017, 152, 1319–1328.e7. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.; Song, S.; Cho, H.N.; Park, B.; Jun, J.K.; Choi, E.; Kim, Y.; Choi, K.S. Trends in Participation Rates for the National Cancer Screening Program in Korea, 2002-2012. Cancer Res. Treat. 2017, 49, 798–806. [Google Scholar] [CrossRef]
- Cooksley, T.; Haji-Michael, P. Oncologic Sepsis on the ICU: Two Decades of Improving Outcomes. Crit. Care Med. 2020, 48, 925–926. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Cooksley, T.; Haji-Michael, P. Short-and long-term outcomes of patients with solid tumours following non-surgical intensive care admission. QJM Int. J. Med. 2018, 111, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-R.; Lai, C.-L.; Chan, K.A. Intensive Care Unit Admission and Survival in Stage IV Cancer Patients with Septic Shock: A Population-Based Cohort Study. J. Cancer 2019, 10, 3179. [Google Scholar] [CrossRef]


| Characteristic | Total (n = 43,466) | Survivor (n = 20,827) | Non-Survivor (n = 22,639) | Univariate Analysis | ||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | p Value | ||||
| Age, years | ||||||
| 18–29 | 397 (0.9%) | 256 (1.2%) | 141 (0.6%) | Reference | <0.001 | |
| 30–39 | 828 (1.9%) | 463 (2.2%) | 365 (1.6%) | 1.344 | 1.106–1.632 | 0.003 |
| 40–49 | 2786 (6.4%) | 1408 (6.8%) | 1378 (6.1%) | 1.576 | 1.325–1.874 | <0.001 |
| 50–59 | 7300 (16.8%) | 3633 (17.4%) | 3667 (16.2%) | 1.630 | 1.378–1.929 | <0.001 |
| 60–69 | 11,772 (27.1%) | 5875 (28.2%) | 5897 (26.1%) | 1.634 | 1.382–1.931 | <0.001 |
| 70–79 | 13,576 (31.2%) | 6187 (29.7%) | 7389 (32.6%) | 1.841 | 1.559–2.175 | <0.001 |
| ≥80 | 6807 (15.7%) | 3005 (14.4%) | 3802 (16.8%) | 1.909 | 1.614–2.259 | <0.001 |
| Female | 15,399 (35.4%) | 7736 (37.1%) | 7663 (33.9%) | 0.909 | 0.884–0.934 | <0.001 |
| Comorbidities | ||||||
| Hypertension | 23,136 (53.2%) | 10,790 (51.8%) | 12,346 (54.5%) | 1.078 | 1.050–1.107 | <0.001 |
| Diabetes | 16,977 (39.1%) | 7828 (37.6%) | 9149 (40.4%) | 1.079 | 1.051–1.108 | <0.001 |
| Congestive heart failure | 5745 (13.2%) | 2540 (12.2%) | 3205 (14.2%) | 1.123 | 1.082–1.166 | <0.001 |
| Chronic lung disease | 5008 (11.5%) | 2084 (10.0%) | 2924 (12.9%) | 1.212 | 1.166–1.260 | <0.001 |
| Renal failure | 2783 (6.4%) | 1262 (6.1%) | 1521 (6.7%) | 1.071 | 1.017–1.129 | 0.009 |
| Liver cirrhosis | 4974 (11.4%) | 1900 (9.1%) | 3074 (13.6%) | 1.364 | 1.313–1.417 | <0.001 |
| Charlson comorbidity index | ||||||
| 0–2 | 7816 (18.0%) | 4731 (22.7%) | 3085 (13.6%) | Reference | <0.001 | |
| 3–4 | 9652 (22.2%) | 4922 (23.6%) | 4730 (20.9%) | 1.366 | 1.306–1.430 | <0.001 |
| 5–7 | 8256 (19.0%) | 3986 (19.1%) | 4270 (18.9%) | 1.469 | 1.402–1.538 | <0.001 |
| ≥8 | 17,742 (40.8%) | 7188 (34.5%) | 10,554 (46.6%) | 1.822 | 1.750–1.896 | <0.001 |
| Cancer type | ||||||
| Brain | 772 (1.8%) | 500 (2.4%) | 272 (1.2%) | Reference | <0.001 | |
| Lung | 6657 (15.3%) | 2469 (11.9%) | 4188 (18.5%) | 2.292 | 2.028–2.591 | <0.001 |
| Liver | 6238 (14.4%) | 2506 (12.0%) | 3732 (16.5%) | 2.160 | 1.909–2.442 | <0.001 |
| Colon | 4494 (10.3%) | 2611 (12.5%) | 1883 (8.3%) | 1.310 | 1.154–1.488 | <0.001 |
| Stomach | 3684 (8.5%) | 1819 (8.7%) | 1865 (8.2%) | 1.677 | 1.477–1.905 | <0.001 |
| Gall bladder | 1981 (4.6%) | 1117 (5.4%) | 864 (3.8%) | 1.336 | 1.166–1.532 | <0.001 |
| Pancreas | 1943 (4.5%) | 913 (4.4%) | 1030 (4.6%) | 1.782 | 1.559–2.037 | <0.001 |
| Leukemia | 1917 (4.4%) | 864 (4.2%) | 1053 (4.7%) | 1.822 | 1.594–2.082 | <0.001 |
| Non-Hodgkin’s lymphoma | 1475 (3.4%) | 742 (3.6%) | 733 (3.2%) | 1.583 | 1.378–1.820 | <0.001 |
| Female reproductive system | 1249 (2.9%) | 740 (3.6%) | 509 (2.3%) | 1.281 | 1.106–1.485 | 0.001 |
| Breast | 1112 (2.6%) | 574 (2.8%) | 538 (2.4%) | 1.623 | 1.402–1.877 | <0.001 |
| Kidney/bladder | 1095 (2.5%) | 567 (2.7%) | 528 (2.3%) | 1.556 | 1.344–1.801 | <0.001 |
| Multiple myeloma | 923 (2.1%) | 446 (2.1%) | 477 (2.1%) | 1.712 | 1.475–1.987 | <0.001 |
| Male reproductive system | 754 (1.7%) | 356 (1.7%) | 398 (1.8%) | 1.817 | 1.558–2.120 | <0.001 |
| Oropharynx | 439 (1.0%) | 242 (1.2%) | 197 (0.9%) | 1.458 | 1.214–1.751 | <0.001 |
| Esophagus | 391 (0.9%) | 190 (0.9%) | 201 (0.9%) | 1.681 | 1.401–2.017 | <0.001 |
| Thyroid | 169 (0.4%) | 101 (0.5%) | 68 (0.3%) | 1.220 | 0.936–1.592 | 0.14 |
| Larynx | 149 (0.3%) | 82 (0.4%) | 67 (0.3%) | 1.399 | 1.071–1.827 | 0.01 |
| Hodgkin lymphoma | 50 (0.1%) | 23 (0.1%) | 27 (0.1%) | 1.642 | 1.106–2.439 | 0.01 |
| Other, unspecified | 2995 (6.9%) | 1622 (7.8%) | 1373 (6.1%) | 1.442 | 1.266–1.642 | <0.001 |
| Multiple | 4979 (11.5%) | 2343 (11.3%) | 2636 (11.6%) | 1.788 | 1.578–2.026 | <0.001 |
| Characteristics | Adjusted Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Hospitalization year | |||
| 2009 | Reference | <0.001 | |
| 2010 | 0.995 | 0.938–1.054 | 0.86 |
| 2011 | 1.029 | 0.970–1.092 | 0.34 |
| 2012 | 1.011 | 0.952–1.074 | 0.72 |
| 2013 | 1.026 | 0.962–1.095 | 0.44 |
| 2014 | 1.026 | 0.958–1.099 | 0.46 |
| 2015 | 0.964 | 0.898–1.035 | 0.31 |
| 2016 | 0.793 | 0.754–0.835 | <0.001 |
| 2017 | 0.788 | 0.750–0.828 | <0.001 |
| Age, years | |||
| 18–29 | Reference | <0.001 | |
| 30–39 | 1.245 | 1.025–1.512 | 0.03 |
| 40–49 | 1.406 | 1.182–1.672 | <0.001 |
| 50–59 | 1.437 | 1.214–1.701 | <0.001 |
| 60–69 | 1.418 | 1.200–1.677 | <0.001 |
| 70–79 | 1.640 | 1.388–1.938 | <0.001 |
| ≥80 | 1.845 | 1.560–2.184 | <0.001 |
| Female | 0.910 | 0.885–0.935 | <0.001 |
| Charlson comorbidity Index | |||
| 0–2 | Reference | <0.001 | |
| 3–4 | 1.373 | 1.312–1.437 | <0.001 |
| 5–7 | 1.455 | 1.389–1.524 | <0.001 |
| ≥8 | 1.861 | 1.787–1.938 | <0.001 |
| Cancer Type | Period | 30-Day Mortality | Period | 1-Year Mortality | ||||
|---|---|---|---|---|---|---|---|---|
| Change Year | APC (95% CI) | AAPC (95% CI) | Change Year | APC (95% CI) | AAPC (95% CI) | |||
| Lung cancer | 2009–2017 | None | −1.7 (−2.7, −0.7) 1 | −1.7 (−2.7, −0.7) 1 | 2009–2017 | None | −0.7 (−0.9, −0.5) 1 | −0.7 (−0.9, −0.5) 1 |
| Hepatobiliary cancer | 2009–2017 | −2.8 (−5.5, 0.0) 1 | 2009–2017 | None | −1.3 (−1.7, −0.8) 1 | −1.3 (−1.7, −0.8) 1 | ||
| 2009–2014 | 2014 | +0.5 (−3.1, +4.3) | ||||||
| 2014–2017 | −8.0 (−15.7, +0.3) | |||||||
| Colon cancer | 2009–2017 | −1.6 (−4.9, +1.8) | 2009–2017 | −1.4 (−2.9, 0.0) 1 | ||||
| 2009–2011 | 2011 | +9.3 (−8.5, +30.5) | 2009–2011 | 2011 | 2.2 (−5.4, +10.3) | |||
| 2011–2017 | −5.0 (−7.4, −2.6) 1 | 2011–2017 | −2.6 (−3.8, −1.5) 1 | |||||
| Stomach cancer | 2009–2017 | None | −2.0 (−3.2, −0.9) 1 | −2.0 (−3.2, −0.9) 1 | 2009–2017 | None | −1.3 (−1.8, −0.7) 1 | −1.3 (−1.8, −0.7) 1 |
| Pancreas cancer | 2009–2017 | −2.1 (−5.8, +1.8) | 2009–2017 | None | −0.7 (−1.6, +0.1) | −0.7 (−1.6, +0.1) | ||
| 2009–2014 | 2014 | +3.7 (−2.3, +10.0) | ||||||
| 2014–2017 | −11.0 (−20.0, −0.8) 1 | |||||||
| Leukemia | 2009–2017 | None | −2.2 (−4.7, +0.3) | −2.2 (−4.7, +0.3) | 2009–2017 | None | −1.5 (−2.4, −0.6) 1 | −1.5 (−2.4, −0.6) 1 |
| Non-Hodgkin lymphoma | 2009–2017 | None | −1.4 (−4.0, +1.4) | −1.4 (−4.0, +1.4) | 2009–2017 | None | −1.1 (−2.3, +0.1) | −1.1 (−2.3, +0.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-J.; Kim, M.-J.; Kim, Y.-J.; Kim, W.Y. Short and Long-Term Mortality Trends for Cancer Patients with Septic Shock Stratified by Cancer Type from 2009 to 2017: A Population-Based Cohort Study. Cancers 2021, 13, 657. https://doi.org/10.3390/cancers13040657
Kim Y-J, Kim M-J, Kim Y-J, Kim WY. Short and Long-Term Mortality Trends for Cancer Patients with Septic Shock Stratified by Cancer Type from 2009 to 2017: A Population-Based Cohort Study. Cancers. 2021; 13(4):657. https://doi.org/10.3390/cancers13040657
Chicago/Turabian StyleKim, Youn-Jung, Min-Ju Kim, Ye-Jee Kim, and Won Young Kim. 2021. "Short and Long-Term Mortality Trends for Cancer Patients with Septic Shock Stratified by Cancer Type from 2009 to 2017: A Population-Based Cohort Study" Cancers 13, no. 4: 657. https://doi.org/10.3390/cancers13040657