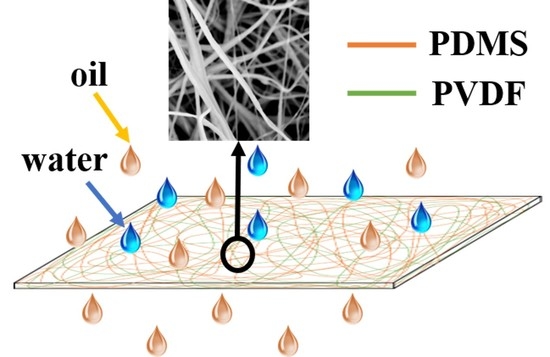
PDMS/PVDF Electrospinning Membranes for Water-in-Oil Emulsion Separation and UV Protection
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of PVDF/PDMS Electrospinning Membrane
2.3. Preparation of Water-in-Oil Emulsion
2.4. Characterization
3. Results and Discussion
3.1. Morphology and Chemical Composition
3.2. Wetting Behavior
3.3. Mechanical Properties and UV Resistance
3.4. Separation Ability of Water-in-Oil Emulsions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, J.; Zhao, H.Y.; Zhu, H.W.; Huang, J.; Shi, L.A.; Yu, S.H. Advanced Sorbents for Oil-Spill Cleanup: Recent Advances and Future Perspectives. Adv. Mater. 2016, 28, 10459–10490. [Google Scholar] [CrossRef]
- Miao, W.; Jiao, D.; Wang, C.; Han, S.; Shen, Q.; Wang, J.; Han, X.; Hou, T.; Liu, J.; Zhang, Y. Ethanol-induced one-step fabrication of superhydrophobic-superoleophilic poly(vinylidene fluoride) membrane for efficient oil/water emulsions separation. J. Water Process. Eng. 2020, 34, 101121. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Zhang, X.; Tang, Y.; Zhou, X.; Zhang, K.; Chen, Z.; Lai, Y. Rational design of materials interface at nanoscale towards intelligent oil–water separation. Nanoscale Horiz. 2018, 3, 235–260. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, J.; Li, S.; Ge, M.; Teng, L.; Chen, Z.; Lai, Y. Advanced Materials with Special Wettability toward Intelligent Oily Wastewater Remediation. ACS Appl. Mater. Interfaces 2021, 13, 67–87. [Google Scholar] [CrossRef]
- Sun, D.; Liu, M.; Guo, J.; Zhang, J.; Li, B.; Li, D. Preparation and characterization of PDMS-PVDF hydrophobic microporous membrane for membrane distillation. Desalination 2015, 370, 63–71. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, J.; Yang, X.; Dong, Y.; Feng, T.; Liu, J. Enhancing the PVA fiber-matrix interface properties in ultra high performance concrete: An experimental and molecular dynamics study. Constr. Build. Mater. 2021, 285, 122862. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Wang, Y.; Zhang, Q.; Ma, W.; Huang, C. Electrospun nanofiber membranes for wastewater treatment applications. Sep. Purif. Technol. 2020, 250, 117116. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Electrospinning of Cyclodextrin Functional Nanofibers for Drug Delivery Applications. Pharmaceutics 2019, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Yao, J.; Sun, H.; Liu, B.; Li, D.; Agtmaal, S.; Feng, C. Preparation and characterization of SiO2/PDMS/PVDF composite membrane for phenols recovery from coal gasification wastewater in pervaporation. Chem. Eng. Res. Des. 2018, 132, 424–435. [Google Scholar] [CrossRef]
- Liao, Y.; Zheng, G.; Huang, J.J.; Tian, M.; Wang, R. Development of robust and superhydrophobic membranes to mitigate membrane scaling and fouling in membrane distillation. J. Membr. Sci. 2020, 601, 117962. [Google Scholar] [CrossRef]
- Tsai, Y.; Maggay, I.V.; Venault, A.; Lin, Y. Fluorine-free and hydrophobic/oleophilic PMMA/PDMS electrospun nanofibrous membranes for gravity-driven removal of water from oil-rich emulsions. Sep. Purif. Technol. 2021, 279, 119720. [Google Scholar] [CrossRef]
- Cheng, C.; Wei, Z.; Gu, J.; Wu, Z.; Zhao, Y. Rational design of Janus nanofibrous membranes with novel under-oil superhydrophilic/superhydrophobic asymmetric wettability for water-in-diesel emulsion separation. J. Colloid Interface Sci. 2022, 606, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhan, H.; Li, D.; Tian, H.; Chang, C. Tunicate cellulose nanocrystals modified commercial filter paper for efficient oil/water separation. J. Membr. Sci. 2019, 591, 117362. [Google Scholar] [CrossRef]
- Han, X.; Guo, Z. Graphene and its derivative composite materials with special wettability: Potential application in oil-water separation. Carbon 2021, 172, 647–681. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, D.; Wei, Z.; Chen, J.; Jing, J. Fabrication of superhydrophobic nano-aluminum films on stainless steel meshes by electrophoretic deposition for oil-water separation. Appl. Surf. Sci. 2018, 427, 253–261. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Yu, X.; Zhao, X.; Zeng, X.; Xu, F.; Tang, X.; Sun, Y.; Lin, L. Facile fabrication of super-hydrophilic cellulose hydrogel-coated mesh using deep eutectic solvent for efficient gravity-driven oil/water separation. Cellulose 2021, 28, 949–960. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Yang, J.; Yue, Y.; Zhang, H. A Review of Recent Advances in Superhydrophobic Surfaces and Their Applications in Drag Reduction and Heat Transfer. Nanomaterials 2022, 12, 44. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Z.; Jiang, G.; Luo, X.; Chen, C.; Hu, X.; Zhang, H.; Zhong, M. Spontaneous dewetting transitions of droplets during icing & melting cycle. Nat. Commun. 2022, 13, 378. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; He, C. Enhanced antifouling performance of hybrid PVDF ultrafiltration membrane with the dual-mode SiO2-g-PDMS nanoparticles. Sep. Purif. Technol. 2016, 166, 1–8. [Google Scholar] [CrossRef]
- Doan, H.N.; Vo, P.P.; Hayashi, K.; Kinashi, K.; Sakai, W.; Tsutsumi, N. Recycled PET as a PDMS-Functionalized electrospun fibrous membrane for oil-water separation. J. Environ. Chem. Eng. 2020, 8, 103921. [Google Scholar] [CrossRef]
- Perween, S.; Khan, Z.; Singh, S.; Ranjan, A. PVA-PDMS-Stearic acid composite nanofibrous mats with improved mechanical behavior for selective filtering applications. Sci. Rep. 2018, 8, 16038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhyou, J.; Youn, J.; Eom, S.; Kim, D.S. Facile Fabrication of Electrospun Nanofiber Membrane-Integrated PDMS Microfluidic Chip via Silver Nanowires-Uncured PDMS Adhesive Layer. ACS Macro Lett. 2021, 10, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Ren, L.F.; Li, J.; Shao, J.; Jawad, A.; Su, C.; Wang, Y.; Guo, L.; He, Y. Enhanced mechanical properties of PDMS/PMMA composite membrane using MWCNTs and its application in phenol separation from saline wastewater. Appl. Polym. 2019, 136, 47123. [Google Scholar] [CrossRef]
- Watanabe, K.; Maeda, T.; Hotta, A. Uniformly dispersed polymeric nanofiber composites by electrospinning: Poly(vinyl alcohol) nanofibers/polydimethylsiloxane composites. Compos. Sci. Technol. 2018, 165, 18–23. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, W.; Chang, C. Environmentally sustainable, fluorine-free and waterproof breathable PDMS/PS nanofibrous membranes for carbon dioxide capture. J. Mater. Chem. A 2018, 6, 9489–9497. [Google Scholar] [CrossRef]
- Luo, Z.; Li, Y.; Duan, C.; Wang, B. Fabrication of a superhydrophobic mesh based on PDMS/SiO2 nanoparticles/PVDF microparticles/KH-550 by one-step dip-coating method. RSC Adv. 2018, 8, 16251–16259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Tang, Y.; Li, B. Excellent wetting resistance and anti-fouling performance of PVDF membrane modified with superhydrophobic papillae-like surfaces. J. Membr. Sci. 2017, 540, 401–410. [Google Scholar] [CrossRef]
- Xiong, J.; Shao, W.; Wang, L.; Cui, C.; Gao, Y.; Jin, Y.; Yu, H.; Han, P.; Liu, F.; He, J. High-performance anti-haze window screen based on multiscale structured polyvinylidene fluoride nanofibers. J. Colloid Interface Sci. 2022, 607, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.F.; Zhang, H. Recent advances in polymer blend membranes for gas separation and pervaporation. Prog. Mater. Science 2021, 116, 100713. [Google Scholar] [CrossRef]
- Pan, Y.; Hang, Y.; Zhao, X.; Liu, G.; Jin, W. Optimizing separation performance and interfacial adhesion of PDMS/PVDF composite membranes for butanol recovery from aqueous solution. J. Membr. Sci. 2019, 579, 210–218. [Google Scholar] [CrossRef]
- Yang, X.; Li, P.; Wu, B.; Li, H.; Zhou, G. A flexible piezoelectric-triboelectric hybrid nanogenerator in one structure with dual doping enhancement effects. Curr. Appl. Phys. 2021, 32, 50–58. [Google Scholar] [CrossRef]
- Moon, C.H.; Yasmeen, S.; Park, K.; Gaiji, H.; Chung, C.; Kim, H.; Moon, H.; Choi, J.W.; Lee, H. Icephobic Coating through a Self-Formed Superhydrophobic Surface Using a Polymer and Microsized Particles. ACS Appl. Mater. Interfaces 2022, 14, 3334–3343. [Google Scholar] [CrossRef] [PubMed]
- Seraji, S.M.; Gui, H.; Zhang, J.; Guo, Q. Nanophase morphology and crystallization in poly(vinylidene fluoride)/polydimethylsiloxane-block-poly(methyl methacrylate) blends. Polym. Int. 2019, 68, 418–427. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, Q.; Hsu, S.; Chung, T. Reusable fluorocarbon-modified electrospun PDMS/PVDF nanofibrous membranes with excellent CO2 absorption performance. Chem. Eng. J. 2016, 284, 888–895. [Google Scholar] [CrossRef]
- An, A.K.; Guo, J.; Lee, E.; Jeong, S.; Zhao, Y.; Wang, Z.; Leiknes, T. PDMS/PVDF hybrid electrospun membrane with superhydrophobic property and drop impact dynamics for dyeing wastewater treatment using membrane distillation. J. Membr. Sci. 2017, 525, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.Q.; Jiao, Y.; Sun, Z.; Yang, X.; Cheng, Z.; Bai, Q.; Zhang, Y.; Wang, K.; Shao, L. Constructing Scalable Superhydrophobic Membranes for Ultrafast Water-Oil Separation. ACS Nano 2021, 15, 3500–3508. [Google Scholar] [CrossRef]
- Ghafari, E.; Jiang, X.; Lu, N. Surface morphology and beta-phase formation of single polyvinylidene fluoride (PVDF) composite nanofibers. Adv. Compos. Hybrid Mater. 2018, 1, 332–340. [Google Scholar] [CrossRef]
- Singh, R.K.; Lye, S.W.; Miao, J. Holistic investigation of the electrospinning parameters for high percentage of β-phase in PVDF nanofibers. Polymer 2021, 214, 123366. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, R.; Li, Y.; Dan, N.; Yang, C.; Yu, G.; Huang, Y.; Wen, H.; Dan, W. Development and analysis of a novel PVDF membrane with higher content of β phase. Int. J. Polym. Anal. Charact. 2019, 24, 684–695. [Google Scholar] [CrossRef]
- Meng, N.; Ren, X.; Santagiuliana, G.; Ventura, L.; Zhang, H.; Wu, J.; Yan, H.; Reece, M.J.; Bilotti, E. Ultrahigh β-phase content poly(vinylidene fluoride) with relaxor-like ferroelectricity for high energy density capacitors. Nat. Commun. 2019, 10, 4535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Jiao, S.; Wang, B.; Tan, Y.; Zhao, Y.; Zhang, Q.; Kang, Y.; Lv, X.; Cui, C.; Pang, G. MoS2/CuS nanosheets coated on brass mesh with switchable superwettability for efficient immiscible organic solvent/water separation. Appl. Surf. Sci. 2021, 570, 151128. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Tran, H.N.; Plamondon, C.O.; Tuduri, L.; Vo, D.N.; Nanda, S.; Mishra, A.; Chao, H.; Bajpai, A.K. Recent progress in the preparation, properties and applications of superhydrophobic nano-based coatings and surfaces: A review. Prog. Org. Coat. 2019, 132, 235–256. [Google Scholar] [CrossRef]
- Nauruzbayeva, J.; Sun, Z.; Gallo, A.; Ibrahim, M.; Santamarina, J.C.; Mishra, H. Electrification at water–hydrophobe interfaces. Nat. Commun. 2020, 11, 5285. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Yuan, X.; Wan, J.; Wang, L.; Pan, H.; Shen, Y. One-pot preparation of robust, ultraviolet-proof superhydrophobic cotton fabrics for self-cleaning and oil/water separation. Cellulose 2020, 27, 9005–9026. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, H.; Qiu, J. Fabrication of biomimetic robust self-cleaning superhydrophobic wood with canna-leaf-like micro/nanostructure through morph-genetic method improved water-, UV-, and corrosion resistance properties. J. Mol. Struct. 2020, 1219, 128616. [Google Scholar] [CrossRef]
- Yu, M.; Liu, M.; Hou, Y.; Fu, S.; Zhang, L.; Li, M.; Wang, D. Covalently grafted liquids for transparent and omniphobic surfaces via thiol-ene click chemistry. J. Mater. Sci. 2020, 55, 12811–12825. [Google Scholar] [CrossRef]









| Max Pulling Force (N) | Elongation (%) | Fracture Strength (MPa) | |
|---|---|---|---|
| P0 | 0.91 | 36.93 | 0.97 |
| P1 | 1.75 | 81.18 | 0.54 |
| P2 | 5.07 | 86.15 | 1.15 |
| P3 | 2.64 | 68.30 | 1.84 |
| P4 | 2.56 | 66.10 | 1.82 |
| P5 | 0.48 | 103.85 | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, Y.; Lu, Y.; Shi, W.; Tian, H. PDMS/PVDF Electrospinning Membranes for Water-in-Oil Emulsion Separation and UV Protection. Biomimetics 2022, 7, 217. https://doi.org/10.3390/biomimetics7040217
Li J, Li Y, Lu Y, Shi W, Tian H. PDMS/PVDF Electrospinning Membranes for Water-in-Oil Emulsion Separation and UV Protection. Biomimetics. 2022; 7(4):217. https://doi.org/10.3390/biomimetics7040217
Chicago/Turabian StyleLi, Jie, Yushan Li, Yiyi Lu, Wentian Shi, and Huafeng Tian. 2022. "PDMS/PVDF Electrospinning Membranes for Water-in-Oil Emulsion Separation and UV Protection" Biomimetics 7, no. 4: 217. https://doi.org/10.3390/biomimetics7040217