Characterization of a Stress-Enhanced Promoter from the Grass Halophyte, Spartina alterniflora L.
Abstract
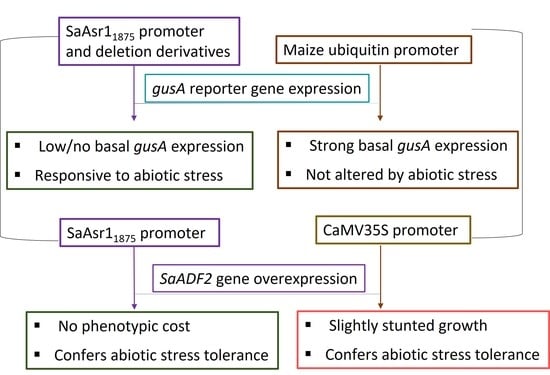
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of the Promoter Region and in Silico Analysis of pASr11875
2.2. Generation of the Promoter: gusA Construct for Transient GUS Expression Assay
2.3. Generation of the Full-Length and Deletion Series Promoter: gusA Constructs and Binary Vector with SaADF2 under the Control of pAsr11875
2.4. Arabidopsis Thaliana Transformation
2.5. Stress Treatment
2.6. Histochemical GUS Staining
2.7. MUG Fluorescence Assay for Quantitative Measurement of gusA Activity
2.8. Semi-Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
3. Results
3.1. Isolation and In-Silico Analysis of the pAsr11875 Promoter
3.2. Stable Reporter Gene Expression in Arabidopsis Transgenic Lines
3.3. Quantitative MUG Assay and gusA Transcript Accumulation under Multiple Abiotic Stresses
3.4. Physiological and Phenotypic Responses of SaADF2 Driven by pAsr11875 and pCaMV35S under Salt and Drought Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dey, N.; Maiti, I. Structure and promoter/leader deletion analysis of mirabilis mosaic virus (MMV) full-length transcript promoter in transgenic plants. Plant Mol. Biol. 1999, 40, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Potenza, C.; Aleman, L.; Sengupta-Gopalan, C. Targeting transgene expression in research, agricultural, and environmental applications: Promoters used in plant transformation. Vitr. Cell. Dev. Biol. Plant 2004, 40, 1–22. [Google Scholar] [CrossRef]
- Somssich, M. A short history of the CaMV 35S promoter. Peer J. Prepr. 2019, 7, e27096v3. [Google Scholar] [CrossRef]
- Peremarti, A.; Twyman, R.M.; Gómez-Galera, S.; Naqvi, S.; Farré, G.; Sabalza, M.; Capell, T. Promoter diversity in multigene transformation. Plant Mol. Biol. 2010, 73, 363–378. [Google Scholar] [CrossRef]
- Koia, J.; Moyle, R.; Hendry, C.; Lim, L.; Botella, J.R. Pineapple translation factor SUI1 and ribosomal protein L36 promoters drive constitutive transgene expression patterns in Arabidopsis thaliana. Plant Mol. Biol. 2013, 81, 327–336. [Google Scholar] [CrossRef]
- Halpin, C. Gene stacking in transgenic plants—The challenge for 21st century plant biotechnology. Plant Biotechnol. J. 2000, 3, 141–155. [Google Scholar] [CrossRef]
- Ahmed, H.A.A.; Onarıcı, S.; Bakhsh, A.; Akdoğan, G.; Karakoç, Ö.C.; Özcan, S.F.; Aydın, G.; Aasim, M.; Ünlü, L.; Sancak, C.; et al. Targeted expression of insecticidal hybrid SN19 gene in potato leads to enhanced resistance against Colorado potato beetle (Leptinotarsa decemlineata Say) and tomato leafminer (Tuta absoluta Meyrick). Plant Biotechnol. Rep. 2017, 11, 315–329. [Google Scholar] [CrossRef]
- Petolino, J.F.; Davies, J.P. Designed transcriptional regulators for trait development. Plant Sci. 2013, 201–202, 128–136. [Google Scholar] [CrossRef]
- Xiao, B.; Huang, Y.; Tang, N.; Xiong, L. Over-expression of a LEA gene in rice improves drought resistance under the field conditions. Theor. Appl. Genet. 2007, 115, 35–46. [Google Scholar] [CrossRef]
- Roslan, N.F.; Rashid, N.S.A.; Suka, I.E.; Taufik, N.A.N.A.; Abdullah, N.S.; Asruri, M.B.; Toni, B.; Sukiran, N.L.; Zainal, Z.; Isa, N. Enhanced tolerance to salinity stress and ABA is regulated by Oryza sativa stress associated protein 8 (OsSAP8). Aust. J. Crop Sci. 2017, 11, 853–860. [Google Scholar] [CrossRef]
- Kim, J.-S.; Mizoi, J.; Yoshida, T.; Fujita, Y.; Nakajima, J.; Ohori, T.; Todaka, D.; Nakashima, K.; Hirayama, T.; Shinozaki, K.; et al. An ABRE promoter sequence is involved in osmotic stress-responsive expression of the DREB2A gene, which encodes a transcription factor regulating drought-inducible genes in Arabidopsis. Plant Cell Physiol. 2011, 52, 2136–2146. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 1994, 6, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Iu, B.; Singh, J. Requirement of a CCGAC cis-acting element for cold induction of the BN115 gene from winter Brassica napus. Plant Mol. Biol. 1996, 30, 679–684. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef] [Green Version]
- Joshi, R.; Ramanarao, M.V.; Bedre, R.; Pilcher, W.; Baisakh, N. Salt adaptation mechanisms of halophytes: Improvement of salt tolerance in crop plants. In Elucidation of Abiotic Stress Signaling in Plants; Pandey, G., Ed.; Springer: New York, NY, USA, 2015; pp. 243–279. [Google Scholar]
- Aslam, R.; Bostan, N.; Maria, M.; Safdar, W. A critical review on halophytes: Salt tolerant plants. J. Med. Plants Res. 2011, 5, 7108–7118. [Google Scholar] [CrossRef]
- Mishra, A.; Tanna, B. Halophytes: Potential resources for salt stress tolerance genes and promoters. Front. Plant Sci. 2017, 8, 829. [Google Scholar] [CrossRef] [PubMed]
- Baisakh, N.; Mangu, V.R. Abiotic stress resistance. Patent number 10465202, 2019. Available online: https://patents.justia.com/patent/10465202 (accessed on 30 November 2022).
- Sengupta, S.; Mangu, V.; Sanchez, L.; Bedre, R.; Joshi, R.; Rajasekaran, K.; Baisakh, N. An actin-depolymerizing factor from the halophyte smooth cordgrass, Spartina alterniflora (SaADF2), is superior to its rice homolog (OsADF2) in conferring drought and salt tolerance when constitutively overexpressed in rice. Plant Biotechnol. J. 2019, 17, 188–205. [Google Scholar] [CrossRef] [Green Version]
- Baisakh, N.; Subudhi, P.K.; Varadwaj, P. Primary responses to salt stress in a halophyte, smooth cordgrass (Spartina alterniflora Loisel.). Funct. Integr. Genom. 2008, 8, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Baisakh, N.; RamanaRao, M.V.; Rajasekaran, K.; Subudhi, P.; Janda, J.; Galbraith, D.; Pereira, A. Enhanced salt stress tolerance of rice plants expressing a vacuolar H+-ATPase subunit c1 (SaVHAc1) gene from the halophyte grass Spartina alterniflora Löisel. Plant Biotechnol. J. 2012, 10, 453–464. [Google Scholar] [CrossRef]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. Plant CARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Mangu, V.R.; Ratnasekera, D.; Yabes, J.C.; Wing, R.A.; Baisakh, N. Functional screening of genes from a halophyte wild rice relative Porteresia coarctata in Arabidopsis model identifies candidate genes involved in salt tolerance. Curr. Plant Biol. 2019, 18, 100107. [Google Scholar] [CrossRef]
- Christensen, A.H.; Quail, P.H. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res. 1996, 5, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Mangu, V.R.; Baisakh, N. Arabidopsis plants constitutively overexpressing a myo-inositol 1-phosphate synthase gene (SaINO1) from the halophyte smooth cordgrass exhibits enhanced level of tolerance to salt stress. Plant Physiol. Biochem. 2013, 65, 61–66. [Google Scholar] [CrossRef]
- Gallagher, S. Gus Protocols: Using the Gus Gene as a Reporter of Gene Expression; Gallagher, S., Ed.; Academic Press: San Diego, CA, USA, 1992; pp. 1–221. [Google Scholar]
- Yanagisawa, S.; Schmidt, R.J. Diversity and similarity among recognition sequences of Dof transcription factors. Plant J. 1999, 17, 209–214. [Google Scholar] [CrossRef]
- Stålberg, K.; Ellerstöm, M.; Ezcurra, I.; Ablov, S.; Rask, L. Disruption of an overlapping E-box/ABRE motif abolished high transcription of the napA storage-protein promoter in transgenic Brassica napus seeds. Planta 1996, 199, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef]
- Rieping, M.; Schöffl, F. Synergistic effect of upstream sequences, CCAAT box elements, and HSE sequences for enhanced expression of chimeric heat shock genes in transgenic tobacco. Mol. Gen. Genet. 1992, 231, 226–232. [Google Scholar] [CrossRef]
- Haralampidis, K.; Trojanowska, M.; Osbourn, A.E. Biosynthesis of triterpenoid saponins in plants. In Adv. Biochem. Eng. Biotechnol. 2002, 75, 32–49. [Google Scholar] [CrossRef]
- Wenkel, S.; Turck, F.; Singer, K.; Gissot, L.; Le Gourrierec, J.; Samach, A.; Coupland, G. CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell 2006, 18, 2971–2984. [Google Scholar] [CrossRef]
- Terzaghi, W.B.; Cashmore, A.R. Light-Regulated Transcription. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 445–474. [Google Scholar] [CrossRef]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Narusaka, Y.; Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Furihata, T.; Abe, H.; Yamaguchi-Shinozaki, K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003, 34, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Yamaguchi-Shinozaki, K.; Urao, T.; Iwasaki, T.; Hosokawa, D.; Shinozaki, K. Role of arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. Plant Cell 1997, 9, 1859–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lata, C.; Prasad, M. Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 2011, 62, 4731–4748. [Google Scholar] [CrossRef] [Green Version]
- Behnam, B.; Iuchi, S.; Fujita, M.; Fujita, Y.; Takasaki, H.; Osakabe, Y.; Shinozaki, K. Characterization of the promoter region of an Arabidopsis gene for 9-cis-epoxycarotenoid dioxygenase involved in dehydration-inducible transcription. DNA Res. 2013, 20, 315–324. [Google Scholar] [CrossRef]





| Matrix Information | Anchor Position (+) | Sequence |
|---|---|---|
| R2R3-type myb-like transcription factor (I-type binding site) | 93 | gttcgtgcCAGTtgccaattt |
| Calmodulin-binding NAC protein | 152 | ctttGCTTtttctttgtgctt |
| Myb domain protein 99 (ATMYBCU15) | 165 | ttgtgcttgagcTAGGtgctc |
| Myb domain protein 46 | 169 | gcttgagcTAGGtgctcccta |
| Homeobox protein 34 | 209 | tatcttttTAATgaaatac |
| Homeodomain GLABROUS 1 | 209 | atctttttAATGaaata |
| Calmodulin-binding transcription activator 1 (AtSR2) | 223 | ataCGCGctcatatgtg |
| MADS-box protein SQUAMOSA | 243 | gttcgctaaAAAAagtttttc |
| KH and zinc finger CCCH domain-containing protein | 245 | aaaaAAAGttt |
| Dof zinc finger protein DOF5.4 (OBF binding protein 4) | 246 | tcgctaaaaAAAGtttttcgggg |
| NAC WITH TRANSMEMBRANE MOTIF 1-LIKE 8 | 275 | agttctcacaagtagaGGAAagg |
| DOF Affecting Germination 2 | 300 | gagacaaatAAAGttggaacatt |
| Heat shock transcription factor C1 | 330 | atctcgttttCAAGaagctatta |
| NAC domain-containing protein 92 (NAC6) | 330 | acatctcgttttcaaGAAGctattaag |
| AP2/ERF and B3 domain-containing transcription factor RAV1 | 380 | atcAACAcagatt |
| GT1-Box binding factors with a trihelix DNA-binding domain | 402 | ttgtgtgtgGTTAcaatag |
| Myb domain protein 96 (MYBCOV1) | 402 | tttgtgtgtGGTTacaataga |
| Early Flowering MYB Protein (AT2G03500) | 462 | ttgaatATTCtaa |
| Heat shock transcription factor B2A | 466 | cttgaataTTCTaaattagcatc |
| I-Box in rbcS genes and other light-regulated genes | 482 | catcaTATAagaatatc |
| MYB protein from wheat | 500 | tgtcatATATtccttgtca |
| Auxin Response Element | 508 | cctTGTCacactt |
| Myb domain protein 33 | 539 | gtgcgtttcaGTTTcaggagg |
| Homeobox-leucine zipper protein ATHB-24 | 555 | gagggaaataATTAataaa |
| Homeobox protein 34 | 559 | gaaataatTAATaaaaaat |
| Homeodomain GLABROUS 1 | 559 | aaataattAATAaaaaa |
| Homeobox-leucine zipper protein ATHB-15 (INCURVATA 4) | 579 | acacataATGAtactcgag |
| Homeobox-leucine zipper protein ATHB-5 | 618 | aaataGAATaattccacat |
| ABA response elements | 625 | taattccACATgtcagt |
| Myb domain protein r1 (ATMYB44) | 632 | ccacatgtcaGTTAtcctaat |
| MybSt1 (Myb Solanum tuberosum 1) with a single myb repeat | 636 | tgtcagttATCCtaataag |
| Homeobox 51, Late Meristem Identity 1 | 671 | aataataaTAATgaattct |
| Arabidopsis thaliana ZF6 (cold-induced zinc finger protein 2) | 699 | gtaaCACTatc |
| Myb domain protein 107 | 740 | gaatgaacagagTTGGttaag |
| MYB-responsive element, MYB46 and MYB83 binding sites | 744 | gaacagagTTGGttaagtgtc |
| Myb-like protein of Petunia hybrida | 745 | aacagagtTGGTtaagtgtct |
| Class I GATA factors | 768 | tcattGATAagacttaa |
| Cis-element in the GAPDH promoters conferring light inducibility | 805 | gcaaATAAagaggaa |
| Dof1/MNB1a—single zinc finger transcription factor | 806 | gatgcaaatAAAGaggaataaat |
| Myb family transcription factor REVEILLE 1 | 817 | ggaataaaTATCttggt |
| Ethylene-responsive elements (ERE) and jasmonate- and elicitor-responsive elements (JERE) | 833 | gtttagagtCGCCgtatcg |
| Ethylene-responsive transcription factor RAP2-6 (secondary DNA binding preference) | 865 | ccaggcaGCCGacaccttc |
| DREB and EAR motif protein 4 (RAP2.10) | 866 | ccaggcagcCGACaccttctc |
| Auxin Response Element | 889 | gtgTGTCccaatt |
| Auxin Response Element | 905 | ccgTGTCaccatc |
| Myb family transcription factor At5g56840 | 920 | ctttcctTTTCcaattgca |
| MybSt1 (Myb Solanum tuberosum 1) with a single myb repeat | 952 | ttacctttATCCaaagtta |
| Myb family transcription factor REVEILLE 1 | 963 | aaagttaaTATCgatgg |
| NAC WITH TRANSMEMBRANE MOTIF 1-LIKE 8 | 968 | agttaatatcgatggaGGAAaag |
| HD-ZIP class III protein ATHB9 | 979 | ggaggaaAAGAttgcaaga |
| Calmodulin-binding NAC protein | 999 | ggttGCTTaagccacaaacaa |
| NAC domain-containing protein 87 | 1001 | aggttgCTTAagccacaaacaacatca |
| Homeodomain protein WUSCHEL | 1066 | actgagATTAatatttctt |
| Oryza sativa CaM-binding transcription factor | 1150 | aatCGTGtactctaggc |
| Homeobox-leucine zipper protein ATHB-53 | 1221 | ggataCAAAaatttagcac |
| Myb domain protein r1 (ATMYB44) | 1271 | gcttcatgcaGTTAtcttttt |
| Arabidopsis NAC domain-containing protein 19 | 1282 | cagttatctttttttTACGgaacatgc |
| NACL-inducible gene 1 | 1286 | tttTTACggaaca |
| NAC domain-containing protein 87 | 1313 | atctttCTTTtttgacgcgaaagcagt |
| WRKY plant-specific zinc-finger-type factor; W box | 1314 | cttttTTGAcgcgaaag |
| Calmodulin-binding transcription activator 1 (AtSR2) | 1320 | tgaCGCGaaagcagtca |
| Arabidopsis thaliana ZF6 (cold-induced zinc finger protein 2) | 1339 | ctaaTACTaat |
| Myb family transcription factor At3g10113 | 1344 | aatactAATAtccgaat |
| Myb family transcription factor At5g61620 | 1346 | atactaaTATCcgaatatt |
| Myb family transcription factor (G2-like family) | 1352 | tatccgaatATTCcaag |
| MYB protein from wheat | 1354 | atccgaATATtccaaggat |
| Heat shock transcription factor B2A | 1357 | tccgaataTTCCaaggattatcc |
| Hordeum vulgare Myb-related CAB-promoter-binding protein 1 | 1366 | caaggattATCCtctgccg |
| Ethylene-responsive transcription factor RAP2-6 (secondary DNA binding preference) | 1374 | atcctctGCCGacagttta |
| DREB and EAR motif protein 4 (RAP2.10) | 1375 | atcctctgcCGACagtttagc |
| NAC domain-containing protein 87 | 1415 | ccatttCTTCgattagaggataaccaa |
| CCAAT-box in plant promoters | 1427 | aaCCAAtgg |
| Ethylene-responsive transcription factor RAP2-6 (secondary DNA binding preference) | 1463 | gggtcctGCCGtccaataa |
| Ethylene-responsive transcription factor ERF018 | 1464 | gggtcctGCCGtccaataact |
| CCAAT-box in plant promoters | 1468 | gtCCAAtaa |
| Homeobox-leucine zipper protein ATHB-53 | 1471 | ccgtcCAATaactgtccgg |
| Ethylene-responsive transcription factor RAP2-6 (secondary DNA binding preference) | 1500 | acttgatGCCGtcatgggc |
| Dehydration-responsive element-binding protein A-4 | 1501 | acttgatgcCGTCatgggcag |
| NAC domain-containing protein 92 (NAC6) | 1507 | tgatgccgtcatgggCAGGcatccgtg |
| MybSt1 (myb Solanum tuberosum 1) with a single myb repeat | 1576 | gctccactATCCatcaatt |
| Homeobox-leucine zipper protein ATHB-23 | 1631 | taatcgtttgATTAatctg |
| Homeodomain protein WUSCHEL | 1635 | cgtttgATTAatctgcaca |
| Homeobox protein 32 | 1665 | caattttaTTATgtactgt |
| Hordeum vulgare Myb-related CAB-promoter-binding protein 1 | 1676 | tgtactgtATCCtttgcta |
| Homeobox protein 33 | 1709 | cagtgtgtTAATcacaatc |
| WUSCHEL-related homeobox 13 | 1716 | tcaCAATcacc |
| Myb-domain transcription factor werewolf | 1729 | accctttgcaGTTTgcaccgt |
| Ethylene-responsive transcription factor ERF017 (AT1G19210) | 1738 | agtttgcACCGtccatcgatc |
| M-phase-specific activators (NtmybA1, NtmybA2, NtmybB) | 1829 | cgtccAACTgtcactgctgtc |
| Basic leucine-zipper 52 | 1841 | gctgtCAGCtctcaa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sengupta, S.; Pehlivan, N.; Mangu, V.; Rajasekaran, K.; Baisakh, N. Characterization of a Stress-Enhanced Promoter from the Grass Halophyte, Spartina alterniflora L. Biology 2022, 11, 1828. https://doi.org/10.3390/biology11121828
Sengupta S, Pehlivan N, Mangu V, Rajasekaran K, Baisakh N. Characterization of a Stress-Enhanced Promoter from the Grass Halophyte, Spartina alterniflora L. Biology. 2022; 11(12):1828. https://doi.org/10.3390/biology11121828
Chicago/Turabian StyleSengupta, Sonali, Necla Pehlivan, Venkata Mangu, Kanniah Rajasekaran, and Niranjan Baisakh. 2022. "Characterization of a Stress-Enhanced Promoter from the Grass Halophyte, Spartina alterniflora L." Biology 11, no. 12: 1828. https://doi.org/10.3390/biology11121828