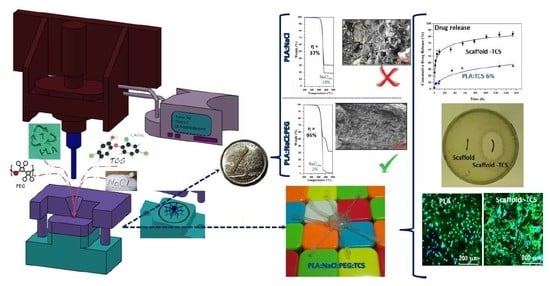
Biodegradable Polylactide Scaffolds with Pharmacological Activity by Means of Ultrasound Micromolding Technology
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Micromolding Equipment and Scaffold Preparation
2.3. Molecular Weight
2.4. FTIR Equipment
2.5. Scanning Electron Microscope
2.6. NMR Measurements
2.7. Surface Contact Angle Measurements
2.8. Calorimetric Assays: DSC and TGA
2.9. Synchrotron Measurements
2.10. Mass Extraction Yield and Porosity Calculations
2.11. Drug Release Experiments
2.12. Antibacterial Assay
2.13. Cell Proliferation Assay
3. Results and Discussion
3.1. Micromolding of Mixtures of PLA and the Porogenic Agent
3.2. Ultrasound Micromolding of PLA/PEG Blends
3.3. Ultrasound Micromolding of PLA:PEG:NaCl Mixtures. Salt Leaching and Scaffold Characterization
3.4. Antibacterial Micromolded Scaffolds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bonassar, L.J.; Vacanti, C.A. Tissue engineering: The first decade and beyond. J. Cell. Biochem. 1998, 72, 297–303. [Google Scholar] [CrossRef]
- Atala, A. Tissue Engineering and Regenerative Medicine: Concepts for Clinical Application. Rejuvenation Res. 2004, 7, 15–31. [Google Scholar] [CrossRef]
- Peter, S.J.; Miller, M.J.; Yasko, A.W.; Yaszemski, M.J.; Mikos, A.G. Polymer concepts in tissue engineering. J. Biomed. Mater. Res. 1998, 43, 422–427. [Google Scholar] [CrossRef]
- Marler, J.J.; Upton, J.; Langer, R.; Vacanti, J.P. Transplantation of cells in matrices for tissue regeneration. Adv. Drug Deliv. Rev. 1998, 33, 165–182. [Google Scholar] [CrossRef]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Novel Starch-Based Scaffolds for Bone Tissue Engineering: Cytotoxicity, Cell Culture, and Protein Expression. Tissue Eng. 2004, 10, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Heller, C.; Schwentenwein, M.; Russmueller, G.; Varga, F.; Stampfl, J.; Liska, R. Vinyl esters: Low cytotoxicity monomers for the fabrication of biocompatible 3D scaffolds by lithography based additive manufacturing. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 6941–6954. [Google Scholar] [CrossRef]
- Watari, F.; Yokoyama, A.; Saso, F.; Uo, M.; Kawasaki, T. Fabrication and properties of functionally graded dental implant. Compos. Part B Eng. 1997, 28, 5–11. [Google Scholar] [CrossRef]
- Walsh, D.; Furuzono, T.; Tanaka, J. Preparation of porous composite implant materials by in situ polymerization of porous apatite containing ε-caprolactone or methyl methacrylate. Biomaterials 2001, 22, 1205–1212. [Google Scholar] [CrossRef]
- Mizuno, S.; Glowacki, J. Three-dimensional composite of demineralized bone powder and collagen for in vitro analysis of chondroinduction of human dermal fibroblasts. Biomaterials 1996, 17, 1819–1825. [Google Scholar] [CrossRef]
- Su, Z.; Ma, H.; Wu, Z.; Zeng, H.; Li, Z.; Wang, Y.; Liu, G.; Xu, B.; Lin, Y.; Zhang, P.; et al. Enhancement of skin wound healing with decellularized scaffolds loaded with hyaluronic acid and epidermal growth factor. Mater. Sci. Eng. C 2014, 44, 440–448. [Google Scholar] [CrossRef]
- Guo, T.; Zhao, J.; Chang, J.; Ding, Z.; Hong, H.; Chen, J.; Zhang, J. Porous chitosan-gelatin scaffold containing plasmid DNA encoding transforming growth factor-β1 for chondrocytes proliferation. Biomaterials 2006, 27, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Guo, M.; Wang, Z.; Shao, N.; Zhang, P.-b.; Chen, X.; Huang, Y. Degradable 3D printed PLA scaffold with long-term antibacterial activity. ACS Sustain. Chem. Eng. 2018, 6, 2047–2054. [Google Scholar] [CrossRef]
- Wu, J.C.-C.; Ray, S.; Gizdavic-Nikolaidis, M.; Uy, B.; Swift, S.; Jin, J.; Cooney, R.P. Nanostructured bioactive material based on polycaprolactone and polyaniline fiber-scaffolds. Synth. Met. 2014, 198, 41–50. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Velasco, M.; Narváez-Tovar, C.A.; Garzón-Alvarado, D.A. Design, Materials, and Mechanobiology of Biodegradable Scaffolds for Bone Tissue Engineering. Biomed Res. Int. 2015, 2015, 21. [Google Scholar] [CrossRef]
- Jeong, D.; Yun, A.; Kim, J. Mathematical model and numerical simulation of the cell growth in scaffolds. Biomech. Model. Mechanobiol. 2012, 11, 677–688. [Google Scholar] [CrossRef]
- Freed, L.E.; Vunjak-Novakovic, G.; Biron, R.J.; Eagle, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable polymer scaffolds for tissue engineering. Nat. Bio-Technol. 1994, 12, 689–693. [Google Scholar] [CrossRef]
- Liu, X.; Holzwarth, J.M.; Ma, P.X. Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef] [Green Version]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- McMurry, L.M.; Oethinger, M.; Levy, S.B. Triclosan targets lipid synthesis. Nature 1998, 394, 531–532. [Google Scholar] [CrossRef]
- Mikos, A.G.; Bao, Y.; Cima, L.G.; Ingber, D.E.; Vacanti, J.P.; Langer, R. Preparation of poly(glycolic acid) bonded fiber structures for cell attachment and transplantation. J. Biomed. Mater. Res. 1993, 27, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; del Valle, L.J.; Tugushi, D.; Katsarava, R.; Puiggalí, J. New poly(ester urea) derived from l-leucine: Electrospun scaffolds loaded with antibacterial drugs and enzymes. Mater. Sci. Eng. C 2015, 46, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Sin, D.; Miao, X.; Liu, G.; Wei, F.; Chadwick, G.; Yan, C.; Friis, T. Polyurethane (PU) scaffolds prepared by solvent casting/particulate leaching (SCPL) combined with centrifugation. Mater. Sci. Eng. C 2010, 30, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.H.; Kang, S.G.; Lee, J.H. Degradation behavior of hydrophilized PLGA scaffolds prepared by melt-molding particulate-leaching method: Comparison with control hydrophobic one. J. Mater. Sci. Mater. Med. 2006, 17, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Widmer, M.S.; Gupta, P.K.; Lu, L.; Meszlenyi, R.K.; Evans, G.R.D.; Brandt, K.; Savel, T.; Gurlek, A.; Patrick, C.W.; Mikos, A.G. Manufacture of porous biodegradable polymer conduits by an extrusion process for guided tissue regeneration. Biomaterials 1998, 19, 1945–1955. [Google Scholar] [CrossRef]
- Utela, B.; Storti, D.; Anderson, R.; Ganter, M. A review of process development steps for new material systems in three dimensional printing (3DP). J. Manuf. Process. 2008, 10, 96–104. [Google Scholar] [CrossRef]
- Bergmann, C.; Lindner, M.; Zhang, W.; Koczur, K.; Kirsten, A.; Telle, R.; Fischer, H. 3D printing of bone substitute implants using calcium phosphate and bioactive glasses. J. Eur. Ceram. Soc. 2010, 30, 2563–2567. [Google Scholar] [CrossRef]
- Yao, D.; Chen, S.-C.; Kim, B.H. Rapid thermal cycling of injection molds: An overview on technical approaches and applications. Adv. Polym. Technol. 2008, 27, 233–255. [Google Scholar] [CrossRef]
- Michaeli, W.; Opferman, D. Ultrasonic plasticising for micro injection moulding. In Proceedings of the 4M 2006—Second International Conference on Multi-Material Micro Manufacture, Grenoble, France, 20–22 September 2006; pp. 345–348. [Google Scholar]
- Yao, D.; Chen, S.-C.; Kim, B.H. High-frequency proximity heating for injection molding applications. Polym. Eng. Sci. 2006, 46, 938–945. [Google Scholar] [CrossRef]
- Sacristán, M.; Puiggalli, J.; Planta, X.; Morell, M. Effects of ultrasonic vibration on the micro-molding processing of polylactide. Ultrason. Sonochem. 2014, 21, 376–386. [Google Scholar]
- Michaeli, W.; Kamps, T.; Hopmann, C. Manufacturing of polymer micro parts by ultrasonic plasticization and direct injection. Microsyst. Technol. 2011, 17, 243–249. [Google Scholar] [CrossRef]
- Michaeli, W.; Spennermann, A.; Gärtner, R. New plastification concepts for micro injection moulding. Microsyst. Technol. 2002, 8, 55–57. [Google Scholar] [CrossRef]
- Michaeli, W.; Starke, C. Ultrasonic investigations of the thermoplastics injection moulding process. Polym. Test. 2005, 24, 205–209. [Google Scholar] [CrossRef]
- Chen, G.; Guo, S.; Li, H. Ultrasonic improvement of rheological behavior of polystyrene. J. Appl. Polym. Sci. 2002, 84, 2451–2460. [Google Scholar] [CrossRef]
- Planellas, M.; Sacristán, M.; Rey, L.; Olmo, C.; Aymamí, J.; Casas, M.T.; Valle, L.J.d.; Franco, L.; Puiggalí, J. Micro-molding with ultrasonic vibration energy: New method to disperse nanoclays in polymer matrices. Ultrason. Sonochem. 2014, 21, 1557–1569. [Google Scholar] [CrossRef]
- Díaz, A.; Franco, L.; Casas, M.T.; del Valle, L.J.; Aymamí, J.; Olmo, C.; Puiggalí, J. Preparation of micro-molded exfoliated clay nanocomposites by means of ultrasonic technology. J. Polym. Res. 2014, 21, 584. [Google Scholar] [CrossRef]
- Olmo, C.; Amestoy, H.; Casas, M.T.; Martínez, J.C.; Franco, L.; Sarasua, J.-R.; Puiggalí, J. Preparation of Nanocomposites of Poly(ε-caprolactone) and Multi-Walled Carbon Nanotubes by Ultrasound Micro-Molding. Influence of Nanotubes on Melting and Crystallization. Polymers 2017, 9, 322. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Ikada, Y. Medical Applications. In Poly(Lactic Acid); Auras, R., Lim, L.-T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Heller, J. Biodegradable polymers in controlled drug delivery. Crit. Rev. Drug Carr. Syst. 1984, 1, 39–90. [Google Scholar]
- Olmo, C.; Franco, L.; del Valle, L.J.; Puiggalí, J. Preparation of Medicated Polylactide Micropieces by Means of Ultrasonic Technology. Appl. Sci. 2019, 9, 2360. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Li, S.H.; De Wijn, J.R.; Layrolle, P.; De Groot, K. Synthesis of macroporous hydroxyapatite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. 2002, 61, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-S.; Sun Park, M.; Jeon, O.; Yong Choi, C.; Kim, B.-S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Kramschuster, A.; Turng, L.-S. An injection molding process for manufacturing highly porous and interconnected biodegradable polymer matrices for use as tissue engineering scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92B, 366–376. [Google Scholar] [CrossRef]
- Huang, R.; Zhu, X.; Zhao, T.; Wan, A. Preparation of tissue engineering porous scaffold with poly(lactic acid) and polyethylene glycol solution blend by solvent-casting/particulate-leaching. Mater. Res. Express 2014, 1, 45403. [Google Scholar] [CrossRef]
- Wu, D.; Lin, D.; Zhang, J.; Zhou, W.; Zhang, M.; Zhang, Y.; Wang, D.; Lin, B. Selective Localization of Nanofillers: Effect on Morphology and Crystallization of PLA/PCL Blends. Macromol. Chem. Phys. 2011, 212, 613–626. [Google Scholar] [CrossRef]
- Yang, M.; Lin, Y. Measurement and Simulation of Thermal Stability of Poly(Lactic Acid) by Thermogravimetric Analysis. J. Test. Eval. 2009, 37, 364–370. [Google Scholar]
- Han, S.; Kim, C.; Kwon, D. Thermal/oxidative degradation and stabilization of polyethylene glycol. Polymer 1997, 38, 317–323. [Google Scholar] [CrossRef]
- Hassan, M.; Chong, L.; Sultana, N. Wettability and water uptake properties of PLA and PCL/Gelatin-based electrospun. ARPN J. Eng. Appl. Sci. 2016, 11, 23. [Google Scholar]
- Szewczyk, P.K.; Ura, D.P.; Metwally, S.; Knapczyk-Korczak, J.; Gajek, M.; Marzec, M.M.; Bernasik, A.; Stachewicz, U. Roughness and Fiber Fraction Dominated Wetting of Electrospun Fiber-Based Porous Meshes. Polymers 2019, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Zurita, R.; Puiggalí, J.; Rodríguez-Galán, A. Triclosan Release from Coated Polyglycolide Threads. Macromol. Biosci. 2006, 6, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Márquez, Y.; Cabral, T.; Lorenzetti, A.; Franco, L.; Turon, P.; del Valle, L.J.; Puiggalí, J. Incorporation of biguanide compounds into poly(GL)-b-poly(GL-co-TMC-co-CL)-b-poly(GL) monofilament surgical sutures. Mater. Sci. Eng. C 2017, 71, 629–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puliafito, A.; Hufnagel, L.; Neveu, P.; Streichan, S.; Sigal, A.; Fygenson, D.K.; Shraiman, B.I. Collective and single cell behavior in epithelial contact inhibition. Proc. Natl. Acad. Sci. USA 2012, 109, 739–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, W.; Sonam, S.; Beng Saw, T.; Ladoux, B.; Teck Lim, C. Emergent patterns of collective cell migration under tubular confinement. Nat. Commun. 2017, 8, 1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tlili, S.; Gauquelin, E.; Li, B.; Cardoso, O.; Ladoux, B.; Delanoë-Ayari, H.; Graner, F. Collective cell migration without proliferation: Density determines cell velocity and wave velocity. R. Soc. Open Sci. 2018, 5, 172421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
















| Sample | PLA [%-wt] | PEG [%-wt] | NaCl 45 nm [%-wt] | TCS [%-wt] | Molding Parameters a |
|---|---|---|---|---|---|
| PLA | 100 | 0 | 0 | 0 | 24/300/1.2 |
| PLA:NaCl-35 | 65 | 0 | 35 | 0 | 24/400/1.2 |
| PLA:NaCl-40 | 60 | 0 | 40 | 0 | 24/400/1.2 |
| PLA:PEG 85% | 85 | 15 | 0 | 0 | 24/300/1.2 |
| PLA:PEG 70% | 70 | 30 | 0 | 0 | 24/300/1.2 |
| PLA:PEG 60% | 60 | 40 | 0 | 0 | 24/300/1.2 |
| PLA:PEG 50% | 50 | 50 | 0 | 0 | 24/300/1.2 |
| PLA:PEG-10:NaCl-40 b | 50 | 10 | 40 | 0 | 24/400/1.2 |
| PLA:PEG-8:NaCl-36:TCS-6 c | 50 | 8 | 36 | 6 | 24/400/1.2 |
| Sample | 1.56 ppm (-CH3) | 3.64 ppm (-CH2-) | PEG (wt-%) |
|---|---|---|---|
| PLA: PEG 85% P | 4.49 | 0.52 | 8.0 |
| PLA: PEG 85% D | 5.03 | 0.45 | 7.2 |
| PLA: PEG 70% P | 3.65 | 0.82 | 14.4 |
| PLA: PEG 70% D | 4.92 | 0.77 | 10.5 |
| PLA: PEG 50% P | 1.06 | 1.42 | 50.2 |
| PLA: PEG 50% D | 1.20 | 1.22 | 43.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmo, C.; Franco, L.; del Valle, L.J.; Puiggalí, J. Biodegradable Polylactide Scaffolds with Pharmacological Activity by Means of Ultrasound Micromolding Technology. Appl. Sci. 2020, 10, 3106. https://doi.org/10.3390/app10093106
Olmo C, Franco L, del Valle LJ, Puiggalí J. Biodegradable Polylactide Scaffolds with Pharmacological Activity by Means of Ultrasound Micromolding Technology. Applied Sciences. 2020; 10(9):3106. https://doi.org/10.3390/app10093106
Chicago/Turabian StyleOlmo, Cristian, Lourdes Franco, Luis J. del Valle, and Jordi Puiggalí. 2020. "Biodegradable Polylactide Scaffolds with Pharmacological Activity by Means of Ultrasound Micromolding Technology" Applied Sciences 10, no. 9: 3106. https://doi.org/10.3390/app10093106