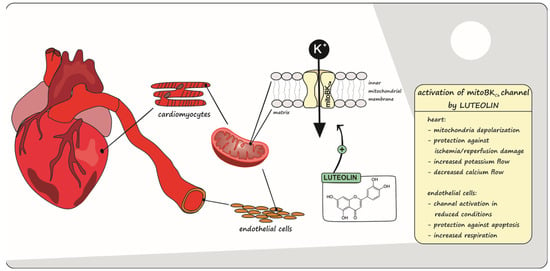
Luteolin-Induced Activation of Mitochondrial BKCa Channels: Undisclosed Mechanism of Cytoprotection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological and Chemical Materials
2.1.1. Animal Model
2.1.2. Cell Culture
2.1.3. Chemical Substances
3. Experimental Methods
3.1. In Vivo Model of Acute Myocardial Infarct
3.2. Cardiac Mitochondria Isolation
3.3. Measurement of Mitochondrial Membrane Potential (ΔΨm)
3.4. Measurements of the Mitochondrial Ca2+ Uptake
3.5. Potassium Cations Flow Measurements
3.6. Mitochondria and Mitoplast Preparation for Electrophysiological Studies
3.7. Patch-Clamp Experiments
3.8. Cell Respiration Measurements
3.9. Apoptosis/Necrosis Assay
3.10. Molecular Modeling Studies
3.11. Statistical Analysis
4. Results
4.1. In Vivo and Ex Vivo Studies
4.1.1. In Vivo Acute Myocardial Infarction and LUT Action
4.1.2. Ex Vivo Changes in Membrane Potential in Cardiac-Isolated Mitochondria
4.1.3. Ex Vivo Calcium Ions Uptake into Cardiac Mitochondria
4.1.4. Ex Vivo Potassium Ions Flow through mitoBKCa Channels in Cardiac Mitochondria
4.2. In Vitro Model of Endothelial Cells: EA.hy926
4.2.1. Regulation of mitoBKCa Activity by LUT under Different Redox Conditions
4.2.2. Impact of Luteolin on Oxygen Consumption
4.2.3. Anti-Apoptotic, Cytoprotective Effects of LUT
4.3. In Silico Studies with LUT and the mitoBKCa Channel
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Rourke, B.; Cortassa, S.; Aon, M.A. Mitochondrial ion channels: Gatekeepers of life and death. Physiology 2005, 20, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.K.; Ponnalagu, D.; Hussain, A.T.; Shah, K.; Karekar, P.; Gururaja Rao, S.; Meredith, A.L.; Khan, M.; Singh, H. Expression and Activation of BKCa Channels in Mice Protects Against Ischemia-Reperfusion Injury of Isolated Hearts by Modulating Mitochondrial Function. Front. Cardiovasc. Med. 2018, 5, 194. [Google Scholar] [CrossRef] [PubMed]
- Honrath, B.; Krabbendam, I.E.; Culmsee, C.; Dolga, A.M. Small conductance Ca(2+)-activated K(+) channels in the plasma membrane, mitochondria and the ER: Pharmacology and implications in neuronal diseases. Neurochem. Int. 2017, 109, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Hu, J.; Xiao, J.; Dan, G.; Yang, L.; Ye, F.; Zou, Z.; Cao, J.; Sai, Y. Mitochondrial ATP-sensitive potassium channel regulates mitochondrial dynamics to participate in neurodegeneration of Parkinson’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1086–1103. [Google Scholar] [CrossRef] [PubMed]
- Garlid, K.D. Opening mitochondrial K(ATP) in the heart—What happens, and what does not happen. Basic Res. Cardiol. 2000, 95, 275–279. [Google Scholar] [CrossRef]
- Soltysinska, E.; Bentzen, B.H.; Barthmes, M.; Hattel, H.; Thrush, A.B.; Harper, M.E.; Qvortrup, K.; Larsen, F.J.; Schiffer, T.A.; Losa-Reyna, J.; et al. KCNMA1 encoded cardiac BK channels afford protection against ischemia-reperfusion injury. PLoS ONE 2014, 9, e103402. [Google Scholar] [CrossRef]
- Borchert, G.H.; Hlaváčková, M.; Kolář, F. Pharmacological activation of mitochondrial BK(Ca) channels protects isolated cardiomyocytes against simulated reperfusion-induced injury. Exp. Biol. Med. 2013, 238, 233–241. [Google Scholar] [CrossRef]
- Szewczyk, A.; Kajma, A.; Malinska, D.; Wrzosek, A.; Bednarczyk, P.; Zabłocka, B.; Dołowy, K. Pharmacology of mitochondrial potassium channels: Dark side of the field. FEBS Lett. 2010, 584, 2063–2069. [Google Scholar] [CrossRef]
- Wrzosek, A.; Augustynek, B.; Żochowska, M.; Szewczyk, A. Mitochondrial Potassium Channels as Druggable Targets. Biomolecules 2020, 10, 1200. [Google Scholar] [CrossRef]
- Wrzosek, A. The potassium channel opener NS1619 modulates calcium homeostasis in muscle cells by inhibiting SERCA. Cell Calcium 2014, 56, 14–24. [Google Scholar] [CrossRef]
- Du, X.; Carvalho-de-Souza, J.L.; Wei, C.; Carrasquel-Ursulaez, W.; Lorenzo, Y.; Gonzalez, N.; Kubota, T.; Staisch, J.; Hain, T.; Petrossian, N.; et al. Loss-of-function BK channel mutation causes impaired mitochondria and progressive cerebellar ataxia. Proc. Natl. Acad. Sci. USA 2020, 117, 6023–6034. [Google Scholar] [CrossRef]
- Toczylowska-Maminska, R.; Olszewska, A.; Laskowski, M.; Bednarczyk, P.; Skowronek, K.; Szewczyk, A. Potassium channel in the mitochondria of human keratinocytes. J. Investig. Dermatol. 2014, 134, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Gáspár, T.; Domoki, F.; Lenti, L.; Katakam, P.V.; Snipes, J.A.; Bari, F.; Busija, D.W. Immediate neuronal preconditioning by NS1619. Brain Res. 2009, 1285, 196–207. [Google Scholar] [CrossRef]
- Kicinska, A.; Kampa, R.P.; Daniluk, J.; Sek, A.; Jarmuszkiewicz, W.; Szewczyk, A.; Bednarczyk, P. Regulation of the Mitochondrial BKCa Channel by the Citrus Flavonoid Naringenin as a Potential Means of Preventing Cell Damage. Molecules 2020, 25, 3010. [Google Scholar] [CrossRef] [PubMed]
- Siemen, D.; Loupatatzis, C.; Borecky, J.; Gulbins, E.; Lang, F. Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem. Biophys. Res. Commun. 1999, 257, 549–554. [Google Scholar] [CrossRef]
- Sato, T.; Saito, T.; Saegusa, N.; Nakaya, H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: A mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation 2005, 111, 198–203. [Google Scholar] [CrossRef]
- Kicinska, A.; Augustynek, B.; Kulawiak, B.; Jarmuszkiewicz, W.; Szewczyk, A.; Bednarczyk, P. A large-conductance calcium-regulated K+ channel in human dermal fibroblast mitochondria. Biochem. J. 2016, 473, 4457–4471. [Google Scholar] [CrossRef]
- Skalska, J.; Piwonska, M.; Wyroba, E.; Surmacz, L.; Wieczorek, R.; Koszela-Piotrowska, I.; Zielinska, J.; Bednarczyk, P.; Dolowy, K.; Wilczynski, G.M.; et al. A novel potassium channel in skeletal muscle mitochondria. Biochim. Biophys. Acta-Bioenerg. 2008, 1777, 651–659. [Google Scholar] [CrossRef]
- Sek, A.; Kampa, R.P.; Kulawiak, B.; Szewczyk, A.; Bednarczyk, P. Identification of the Large-Conductance Ca(2+)-Regulated Potassium Channel in Mitochondria of Human Bronchial Epithelial Cells. Molecules 2021, 26, 3233. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Koziel, A.; Jarmuszkiewicz, W.; Szewczyk, A. Large-conductance Ca(2)(+)-activated potassium channel in mitochondria of endothelial EA.hy926 cells. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1415–H1427. [Google Scholar] [CrossRef] [PubMed]
- Stowe, D.F.; Yang, M.; Heisner, J.S.; Camara, A.K.S. Endogenous and Agonist-induced Opening of Mitochondrial Big Versus Small Ca2+-sensitive K+ Channels on Cardiac Cell and Mitochondrial Protection. J. Cardiovasc. Pharmacol. 2017, 70, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Kampa, R.P.; Gliździńska, A.; Szewczyk, A.; Bednarczyk, P.; Filipek, S. Flavonoid quercetin abolish paxilline inhibition of the mitochondrial BKCa channel. Mitochondrion 2022, 65, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Szabo, I.; Zoratti, M. Mitochondrial channels: Ion fluxes and more. Physiol. Rev. 2014, 94, 519–608. [Google Scholar] [CrossRef]
- Kicinska, A.; Jarmuszkiewicz, W. Flavonoids and Mitochondria: Activation of Cytoprotective Pathways? Molecules 2020, 25, 3060. [Google Scholar] [CrossRef] [PubMed]
- Duluc, L.; Soleti, R.; Clere, N.; Andriantsitohaina, R.; Simard, G. Mitochondria as potential targets of flavonoids: Focus on adipocytes and endothelial cells. Curr. Med. Chem. 2012, 19, 4462–4474. [Google Scholar] [CrossRef] [PubMed]
- Testai, L. Flavonoids and mitochondrial pharmacology: A new paradigm for cardioprotection. Life Sci. 2015, 135, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Heiss, C.; Spencer, J.P.; Keen, C.L.; Lupton, J.R.; Schmitz, H.H. Recommending flavanols and procyanidins for cardiovascular health: Current knowledge and future needs. Mol. Asp. Med. 2010, 31, 546–557. [Google Scholar] [CrossRef]
- Testai, L.; Martelli, A.; Cristofaro, M.; Breschi, M.C.; Calderone, V. Cardioprotective effects of different flavonoids against myocardial ischaemia/reperfusion injury in Langendorff-perfused rat hearts. J. Pharm. Pharmacol. 2013, 65, 750–756. [Google Scholar] [CrossRef]
- Chen, Z.; Kong, S.; Song, F.; Li, L.; Jiang, H. Pharmacokinetic study of luteolin, apigenin, chrysoeriol and diosmetin after oral administration of Flos Chrysanthemi extract in rats. Fitoterapia 2012, 83, 1616–1622. [Google Scholar] [CrossRef]
- Lim, S.H.; Jung, S.K.; Byun, S.; Lee, E.J.; Hwang, J.A.; Seo, S.G.; Kim, Y.A.; Yu, J.G.; Lee, K.W.; Lee, H.J. Luteolin suppresses UVB-induced photoageing by targeting JNK1 and p90RSK2. J. Cell. Mol. Med. 2013, 17, 672–680. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Peng, W.H.; Wu, L.C.; Wu, C.C.; Hsu, S.L. Luteolin ameliorates experimental lung fibrosis both in vivo and in vitro: Implications for therapy of lung fibrosis. J. Agric. Food Chem. 2010, 58, 11653–11661. [Google Scholar] [CrossRef] [PubMed]
- Domitrović, R.; Jakovac, H.; Tomac, J.; Šain, I. Liver fibrosis in mice induced by carbon tetrachloride and its reversion by luteolin. Toxicol. Appl. Pharmacol. 2009, 241, 311–321. [Google Scholar] [CrossRef]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Jin, Y.; Xu, X.; Liang, G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, C.; Zhu, H.; Wang, S.; Zhou, Y.; Zhao, J.; Xia, Y.; Li, D. Luteolin modulates SERCA2a via Sp1 upregulation to attenuate myocardial ischemia/reperfusion injury in mice. Sci. Rep. 2020, 10, 15407. [Google Scholar] [CrossRef]
- Xu, T.; Li, D.; Jiang, D. Targeting cell signaling and apoptotic pathways by luteolin: Cardioprotective role in rat cardiomyocytes following ischemia/reperfusion. Nutrients 2012, 4, 2008–2019. [Google Scholar] [CrossRef]
- Bian, C.; Xu, T.; Zhu, H.; Pan, D.; Liu, Y.; Luo, Y.; Wu, P.; Li, D. Luteolin Inhibits Ischemia/Reperfusion-Induced Myocardial Injury in Rats via Downregulation of microRNA-208b-3p. PLoS ONE 2015, 10, e0144877. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Basili, S.; Paoletti, V.; Davì, G. Endothelial dysfunction and oxidative stress in arterial hypertension. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 222–233. [Google Scholar] [CrossRef]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. Adv. Exp. Med. Biol. 2017, 956, 511–540. [Google Scholar] [CrossRef] [PubMed]
- Edgell, C.J.; McDonald, C.C.; Graham, J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. USA 1983, 80, 3734–3737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderone, V.; Testai, L.; Martelli, A.; Rapposelli, S.; Digiacomo, M.; Balsamo, A.; Breschi, M.C. Anti-ischemic properties of a new spiro-cyclic benzopyran activator of the cardiac mito-KATP channel. Biochem. Pharmacol. 2010, 79, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Martelli, A.; Marino, A.; D’Antongiovanni, V.; Ciregia, F.; Giusti, L.; Lucacchini, A.; Chericoni, S.; Breschi, M.C.; Calderone, V. The activation of mitochondrial BK potassium channels contributes to the protective effects of naringenin against myocardial ischemia/reperfusion injury. Biochem. Pharmacol. 2013, 85, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, P.; Wieckowski, M.R.; Broszkiewicz, M.; Skowronek, K.; Siemen, D.; Szewczyk, A. Putative Structural and Functional Coupling of the Mitochondrial BKCa Channel to the Respiratory Chain. PLoS ONE 2013, 8, e68125. [Google Scholar] [CrossRef]
- Kampa, R.P.; Kicinska, A.; Jarmuszkiewicz, W.; Pasikowska-Piwko, M.; Dolegowska, B.; Debowska, R.; Szewczyk, A.; Bednarczyk, P. Naringenin as an opener of mitochondrial potassium channels in dermal fibroblasts. Exp. Dermatol. 2019, 28, 543–550. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Kampa, R.P.; Gałecka, S.; Sęk, A.; Walewska, A.; Koprowski, P. Patch-Clamp Recording of the Activity of Ion Channels in the Inner Mitochondrial Membrane. Methods Mol. Biol. 2021, 2276, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.H.; Chen, S.Y.; Hsieh, Y.L.; Teng, Y.H.; Cheng, Y.J. Low-level laser therapy prevents endothelial cells from TNF-alpha/cycloheximide-induced apoptosis. Lasers Med. Sci. 2018, 33, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Babu, D.; Soenen, S.J.; Raemdonck, K.; Leclercq, G.; De Backer, O.; Motterlini, R.; Lefebvre, R.A. TNF-α/cycloheximide-induced oxidative stress and apoptosis in murine intestinal epithelial MODE-K cells. Curr. Pharm. Des. 2012, 18, 4414–4425. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; MacKinnon, R. Molecular structures of the human Slo1 K+ channel in complex with β4. eLife 2019, 8, e51409. [Google Scholar] [CrossRef]
- Paventi, G.; Soldovieri, M.V.; Servettini, I.; Barrese, V.; Miceli, F.; Sisalli, M.J.; Ambrosino, P.; Mosca, I.; Vinciguerra, I.; Testai, L.; et al. Kv7.4 channels regulate potassium permeability in neuronal mitochondria. Biochem. Pharmacol. 2022, 197, 114931. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G.; Crompton, M. Mitochondrial calcium transport. FEBS Lett. 1980, 111, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Kampa, R.P.; Sek, A.; Szewczyk, A.; Bednarczyk, P. Cytoprotective effects of the flavonoid quercetin by activating mitochondrial BKCa channels in endothelial cells. Biomed. Pharmacother. 2021, 142, 112039. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.C.; Cox, J.O.; Seashols-Williams, S.J.; Dawson Cruz, T. The effects of dithiothreitol (DTT) on fluorescent qPCR dyes. J. Forensic Sci. 2021, 66, 700–708. [Google Scholar] [CrossRef]
- Ko, J.H.; Ibrahim, M.A.; Park, W.S.; Ko, E.A.; Kim, N.; Warda, M.; Lim, I.; Bang, H.; Han, J. Cloning of large-conductance Ca(2+)-activated K(+) channel alpha-subunits in mouse cardiomyocytes. Biochem. Biophys. Res. Commun. 2009, 389, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Krabbendam, I.E.; Honrath, B.; Culmsee, C.; Dolga, A.M. Mitochondrial Ca(2+)-activated K(+) channels and their role in cell life and death pathways. Cell Calcium 2018, 69, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, M.; Kicinska, A.; Szewczyk, A.; Jarmuszkiewicz, W. Mitochondrial large-conductance potassium channel from Dictyostelium discoideum. Int. J. Biochem. Cell Biol. 2015, 60, 167–175. [Google Scholar] [CrossRef]
- Liao, P.H.; Hung, L.M.; Chen, Y.H.; Kuan, Y.H.; Zhang, F.B.; Lin, R.H.; Shih, H.C.; Tsai, S.K.; Huang, S.S. Cardioprotective effects of luteolin during ischemia-reperfusion injury in rats. Circ. J. 2011, 75, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Q.; Li, D.Y.; Xu, T.D.; Zhu, S.S.; Pan, H.J.; Fang, F.; Wu, X.; Sun, H. Antioxidative effect of luteolin pretreatment on simulated ischemia/reperfusion injury in cardiomyocyte and perfused rat heart. Chin. J. Integr. Med. 2017, 23, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A Flavonoid that Has Multiple Cardio-Protective Effects and Its Molecular Mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Lin, Q.; Ji, Y.G.; Zhao, Y.C.; Ding, L.N.; Zhou, W.J.; Zhang, L.H.; Gao, C.Y.; Zhao, W. Luteolin ameliorates rat myocardial ischaemia-reperfusion injury through activation of peroxiredoxin II. Br. J. Pharmacol. 2018, 175, 3315–3332. [Google Scholar] [CrossRef] [PubMed]
- Calderone, V.; Chericoni, S.; Martinelli, C.; Testai, L.; Nardi, A.; Morelli, I.; Breschi, M.C.; Martinotti, E. Vasorelaxing effects of flavonoids: Investigation on the possible involvement of potassium channels. Naunyn-Schmiedebergs Arch. Pharmacol. 2004, 370, 290–298. [Google Scholar] [CrossRef]
- Jiang, H.; Xia, Q.; Wang, X.; Song, J.; Bruce, I.C. Luteolin induces vasorelaxion in rat thoracic aorta via calcium and potassium channels. Pharmazie 2005, 60, 444–447. [Google Scholar] [PubMed]
- Seo, A.; Jackson, J.L.; Schuster, J.V.; Vardar-Ulu, D. Using UV-absorbance of intrinsic dithiothreitol (DTT) during RP-HPLC as a measure of experimental redox potential in vitro. Anal. Bioanal. Chem. 2013, 405, 6379–6384. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.H.; Yaghmour, M.A.; Lee, M.H.; Gradziel, T.M.; Leveau, J.H.J.; Bostock, R.M. An roGFP2-Based Bacterial Bioreporter for Redox Sensing of Plant Surfaces. Phytopathology 2020, 110, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Rotko, D.; Kunz, W.S.; Szewczyk, A.; Kulawiak, B. Signaling pathways targeting mitochondrial potassium channels. Int. J. Biochem. Cell Biol. 2020, 125, 105792. [Google Scholar] [CrossRef]
- Olschewski, A.; Weir, E.K. Redox regulation of ion channels in the pulmonary circulation. Antioxid. Redox Signal. 2015, 22, 465–485. [Google Scholar] [CrossRef]
- Messias Sandes, J.; Nascimento Moura, D.M.; Divina da Silva Santiago, M.; Barbosa de Lima, G.; Cabral Filho, P.E.; da Cunha Gonçalves de Albuquerque, S.; de Paiva Cavalcanti, M.; Fontes, A.; Bressan Queiroz Figueiredo, R.C. The effects of endoplasmic reticulum stressors, tunicamycin and dithiothreitol on Trypanosoma cruzi. Exp. Cell Res. 2019, 383, 111560. [Google Scholar] [CrossRef]
- Ługowski, M.; Saczko, J.; Kulbacka, J.; Banaś, T. Reactive oxygen and nitrogen species. Pol. Merkur. Lek. 2011, 31, 313–317. [Google Scholar]
- Larosa, V.; Remacle, C. Insights into the respiratory chain and oxidative stress. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Testai, L.; Da Pozzo, E.; Piano, I.; Pistelli, L.; Gargini, C.; Breschi, M.C.; Braca, A.; Martini, C.; Martelli, A.; Calderone, V. The Citrus Flavanone Naringenin Produces Cardioprotective Effects in Hearts from 1 Year Old Rat, through Activation of mitoBK Channels. Front. Pharmacol. 2017, 8, 71. [Google Scholar] [CrossRef]
- Walewska, A.; Krajewska, M.; Stefanowska, A.; Buta, A.; Bilewicz, R.; Krysiński, P.; Bednarczyk, P.; Koprowski, P.; Szewczyk, A. Methods of Measuring Mitochondrial Potassium Channels: A Critical Assessment. Int. J. Mol. Sci. 2022, 23, 1210. [Google Scholar] [CrossRef] [PubMed]
- Walewska, A.; Kulawiak, B.; Szewczyk, A.; Koprowski, P. Mechanosensitivity of mitochondrial large-conductance calcium-activated potassium channels. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Checchetto, V.; Azzolini, M.; Peruzzo, R.; Capitanio, P.; Leanza, L. Mitochondrial potassium channels in cell death. Biochem. Biophys. Res. Commun. 2018, 500, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, A.; Jarmuszkiewicz, W.; Kunz, W.S. Mitochondrial potassium channels. IUBMB Life 2009, 61, 134–143. [Google Scholar] [CrossRef]
- Beavis, A.D.; Lu, Y.; Garlid, K.D. On the regulation of K+ uniport in intact mitochondria by adenine nucleotides and nucleotide analogs. J. Biol. Chem. 1993, 268, 997–1004. [Google Scholar] [CrossRef]
- Heinen, A.; Camara, A.K.S.; Aldakkak, M.; Rhodes, S.S.; Riess, M.L.; Stowe, D.F. Mitochondrial Ca2+-induced K+ influx increases respiration and enhances ROS production while maintaining membrane potential. Am. J. Physiol.-Cell Physiol. 2007, 292, C148–C156. [Google Scholar] [CrossRef]
- Debska, G.; May, R.; Kicińska, A.; Szewczyk, A.; Elger, C.E.; Kunz, W.S. Potassium channel openers depolarize hippocampal mitochondria. Brain Res. 2001, 892, 42–50. [Google Scholar] [CrossRef]
- Testai, L.; Sestito, S.; Martelli, A.; Gorica, E.; Flori, L.; Calderone, V.; Rapposelli, S. Synthesis and pharmacological characterization of mitochondrial K(ATP) channel openers with enhanced mitochondriotropic effects. Bioorg. Chem. 2021, 107, 104572. [Google Scholar] [CrossRef]
- Sun, D.; Huang, J.; Zhang, Z.; Gao, H.; Li, J.; Shen, M.; Cao, F.; Wang, H. Luteolin limits infarct size and improves cardiac function after myocardium ischemia/reperfusion injury in diabetic rats. PLoS ONE 2012, 7, e33491. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Z.; Zhu, C.; Fu, Z.; Yu, D. Luteolin alleviates myocardial ischemia reperfusion injury in rats via Siti1/NLRP3/NF-κB pathway. Int. Immunopharmacol. 2020, 85, 106680. [Google Scholar] [CrossRef]
- Fusi, F.; Trezza, A.; Tramaglino, M.; Sgaragli, G.; Saponara, S.; Spiga, O. The beneficial health effects of flavonoids on the cardiovascular system: Focus on K(+) channels. Pharmacol. Res. 2020, 152, 104625. [Google Scholar] [CrossRef] [PubMed]
- Taheri, Y.; Sharifi-Rad, J.; Antika, G.; Yılmaz, Y.B.; Tumer, T.B.; Abuhamdah, S.; Chandra, S.; Saklani, S.; Kılıç, C.S.; Sestito, S.; et al. Paving Luteolin Therapeutic Potentialities and Agro-Food-Pharma Applications: Emphasis on In Vivo Pharmacological Effects and Bioavailability Traits. Oxid. Med. Cell Longev. 2021, 2021, 1987588. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kampa, R.P.; Flori, L.; Sęk, A.; Spezzini, J.; Brogi, S.; Szewczyk, A.; Calderone, V.; Bednarczyk, P.; Testai, L. Luteolin-Induced Activation of Mitochondrial BKCa Channels: Undisclosed Mechanism of Cytoprotection. Antioxidants 2022, 11, 1892. https://doi.org/10.3390/antiox11101892
Kampa RP, Flori L, Sęk A, Spezzini J, Brogi S, Szewczyk A, Calderone V, Bednarczyk P, Testai L. Luteolin-Induced Activation of Mitochondrial BKCa Channels: Undisclosed Mechanism of Cytoprotection. Antioxidants. 2022; 11(10):1892. https://doi.org/10.3390/antiox11101892
Chicago/Turabian StyleKampa, Rafał P., Lorenzo Flori, Aleksandra Sęk, Jacopo Spezzini, Simone Brogi, Adam Szewczyk, Vincenzo Calderone, Piotr Bednarczyk, and Lara Testai. 2022. "Luteolin-Induced Activation of Mitochondrial BKCa Channels: Undisclosed Mechanism of Cytoprotection" Antioxidants 11, no. 10: 1892. https://doi.org/10.3390/antiox11101892