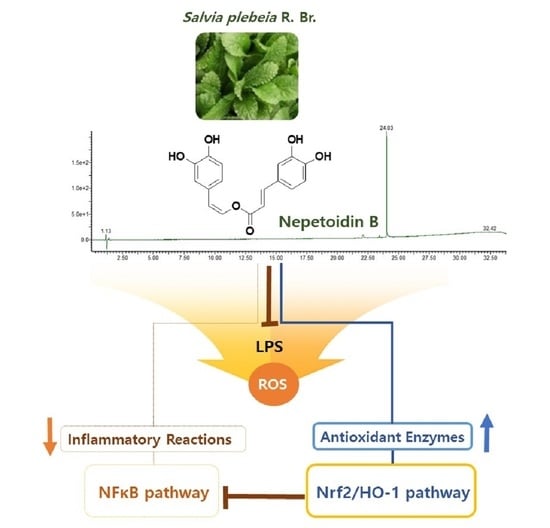
Nepetoidin B from Salvia plebeia R. Br. Inhibits Inflammation by Modulating the NF-κB and Nrf2/HO-1 Signaling Pathways in Macrophage Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Reagents and Instruments
2.3. Extraction and Isolation
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Nitric Oxide Assay
2.7. Measurement of Cytokines
2.8. Intracellular Reactive Oxygen Species Generation
2.9. Antioxidant Enzyme Activity
2.10. NF-κB Binding Activity
2.11. Immunofluorescence Assay
2.12. Western Blot Analysis
2.13. Statistical Analysis
3. Results and Discussion
3.1. Isolation of NeB
3.2. NeB Inhibits the Generation of Pro-Inflammatory Cytokines and Mediators in LPS-Stimulated RAW 264.7 Cells
3.3. NeB Inhibits LPS-Stimulated NF-κB Binding Activity
3.4. NeB Inhibits the LPS-Induced Generation of ROS
3.5. NeB Attenuates Inflammation by Up-Regulating the Nrf-2/HO-1 Key Signaling Pathway
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.C.; Kwon, Y.W.; Park, J.Y.; Park, S.Y.; Lee, J.H.; Park, S.D. Antioxidant and Anti-Inflammatory Effects of Herbal Formula SC-E3 in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Evid. Based. Complement. Alternat. Med. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akram, M.; Syed, A.S.; Kim, K.A.; Lee, J.S.; Chang, S.Y.; Kim, C.Y.; Bae, O.N. Heme oxygenase 1-mediated novel anti-inflammatory activities of Salvia plebeia and its active components. J. Ethnopharmacol. 2015, 174, 322–330. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- So, Y.; Lee, S.Y.; Han, A.R.; Kim, J.B.; Jeong, H.G.; Jin, C.H. Rosmarinic Acid Methyl Ester Inhibits LPS-Induced NO Production via Suppression of MyD88- Dependent and -Independent Pathways and Induction of HO-1 in RAW 264.7 Cells. Molecules 2016, 21, 1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, D.H.; Cha, H.J.; Choi, E.O.; Leem, S.H.; Kim, G.Y.; Moon, S.K.; Chang, Y.C.; Yun, S.J.; Hwang, H.J.; Kim, B.W.; et al. Schisandrin A suppresses lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 macrophages by suppressing the NF-kappaB, MAPKs and PI3K/Akt pathways and activating Nrf2/HO-1 signaling. Int. J. Mol. Med. 2018, 41, 264–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.Q.C.; Duy Binh, T.; Pham, T.L.A.; Nguyen, Y.D.H.; Thi Xuan Trang, D.; Nguyen, T.T.; Kanaori, K.; Kamei, K. Anti-Inflammatory Effects of Lasia spinosa Leaf Extract in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Int. J. Mol. Sci. 2020, 21, 3439. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol. 2012, 3, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.X.; Takano, A.; Drew, B.T.; Liu, E.D.; Soltis, D.E.; Soltis, P.S.; Peng, H.; Xiang, C.L. Phylogeny and staminal evolution of Salvia (Lamiaceae, Nepetoideae) in East Asia. Ann. Bot. 2018, 122, 649–668. [Google Scholar] [CrossRef]
- Choi, J.K.; Oh, H.M.; Lee, S.; Kwon, T.K.; Shin, T.Y.; Rho, M.C.; Kim, S.H. Salvia plebeia suppresses atopic dermatitis-like skin lesions. Am J. Chin. Med. 2014, 42, 967–985. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Wan, X.H.; Niu, F.J.; Xie, S.M.; Guo, H.; Yang, Y.Y.; Guo, L.Y.; Zhou, C.Z. Salvia plebeia R. Br.: An overview about its traditional uses, chemical constituents, pharmacology and modern applications. Biomed. Pharmacother. 2020, 121, 1–18. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, S.; Lee, S.J.; Lim, H.J.; Jung, K.; Kim, Y.H.; Lee, S.W.; Rho, M.C. Anti-inflammatory Activity of Eudesmane-Type Sesquiterpenoids from Salvia plebeia. J. Nat. Prod. 2017, 80, 2666–2676. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Nam, D.G.; Ju, W.T.; Choe, J.S.; Choi, A.J. Response Surface Methodology for Optimization of Process Parameters and Antioxidant Properties of Mulberry (Morus alba L.) Leaves by Extrusion. Molecules 2020, 25, 5231. [Google Scholar] [CrossRef] [PubMed]
- Grayer, R.J.; Eckert, M.R.; Veitch, N.C.; Kite, G.C.; Marin, P.D.; Kokubun, T.; Simmonds, M.S.J.; Paton, A.J. The chemotaxonomic significance of two bioactive caffeic acid esters, nepetoidins A and B, in the Lamiaceae. Phytochemistry 2003, 64, 519–528. [Google Scholar] [CrossRef]
- Wu, X.; Gao, H.; Sun, W.; Yu, J.; Hu, H.; Xu, Q.; Chen, X. Nepetoidin B, a Natural Product, Inhibits LPS stimulated Nitric Oxide Production via Modulation of iNOS Mediated by NF-κB/MKP-5 Pathways. Phytother. Res. 2017, 31, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Inada, M.N.A.; Obata, H.; Tanabe, N.; Abe, S.; Wakashiro, M. Two new potent inhibitors of xanthine oxidase from leaves of Perilla frutescens Britton var. acuta Kudo. Chem. Pharm. Bull. 1990, 38, 1772–1774. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.H.; Zhao, L.; Xu, Y.K.; Bao, J.M.; Liu, X.; Zhang, J.S.; Li, W.; Ahmed, A.; Yin, S.; Tang, G.H. Anti-inflammatory sesquiterpenoids from the Traditional Chinese Medicine Salvia plebeia: Regulates pro-inflammatory mediators through inhibition of NF-kappaB and Erk1/2 signaling pathways in LPS-induced Raw264.7 cells. J. Ethnopharmacol. 2018, 210, 95–106. [Google Scholar] [CrossRef]
- Payne, D.N.R. Nitric oxide in allergic airway inflammation. Curr. Opin. Allergy Clin. Immunol. 2003, 3, 133–137. [Google Scholar] [CrossRef]
- Kacem, M.; Simon, G.; Leschiera, R.; Misery, L.; Elfeki, A.; Lebonvallet, N. Antioxidant and anti-inflammatory effects of Ruta chalepensis L. extracts on LPS-stimulated RAW 264.7 cells. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 128–141. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Sanjeewa, K.K.A.; Nagahawatta, D.P.; Lee, H.G.; Lu, Y.A.; Vaas, A.; Abeytunga, D.T.U.; Nanayakkara, C.M.; Lee, D.S.; Jeon, Y.J. Anti-Inflammatory Effects of Sulfated Polysaccharide from Sargassum Swartzii in Macrophages via Blocking TLR/NF-Kappab Signal Transduction. Mar. Drugs 2020, 18, 601. [Google Scholar] [CrossRef]
- Nasry, W.H.S.; Rodriguez-Lecompte, J.C.; Martin, C.K. Role of COX-2/PGE2 Mediated Inflammation in Oral Squamous Cell Carcinoma. Cancers 2018, 10, 348. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Shi, J.; Wen, H.; Xie, W.; Han, X.; Li, H. Chondroprotective Effect of Moracin on IL-1β-Induced Primary Rat Chondrocytes and an Osteoarthritis Rat Model through Nrf2/HO-1 and NF-κB Axis. Food Funct. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Jang, H.H.; Cho, S.Y.; Kim, M.J.; Kim, J.B.; Lee, S.H.; Lee, M.Y.; Lee, Y.M. Anti-inflammatory effects of Salvia plebeia R. Br extract in vitro and in ovalbumin-induced mouse model. Biol. Res. 2016, 49, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hassanein, E.H.M.; Sayed, A.M.; Hussein, O.E.; Mahmoud, A.M. Coumarins as Modulators of the Keap1/Nrf2/ARE Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.M.; Kim, Y.K.; Bilkis, T.; Suh, J.W.; Lee, D.Y.; Yoo, J.C. Reduction of Oxidative Stress through Activating the Nrf2 mediated HO-1 Antioxidant Efficacy Signaling Pathway by MS15, an Antimicrobial Peptide from Bacillus velezensis. Antioxidants 2020, 9, 934. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajpai, V.K.; Alam, M.B.; Quan, K.T.; Kwon, K.R.; Ju, M.K.; Choi, H.J.; Lee, J.S.; Yoon, J.I.; Majumder, R.; Rather, I.A.; et al. Antioxidant efficacy and the upregulation of Nrf2-mediated HO-1 expression by (+)-lariciresinol, a lignan isolated from Rubia philippinensis, through the activation of p38. Sci. Rep. 2017, 7, 46035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietray polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Yerra, V.G.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 2013, 2013, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Khurana, N.; Sikka, S.C. Targeting Crosstalk between Nrf-2, NF-kappaB and Androgen Receptor Signaling in Prostate Cancer. Cancers 2018, 10, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Kim, J.Y.; Yang, H.S.; Choe, J.-S.; Hwang, I.G. Nepetoidin B from Salvia plebeia R. Br. Inhibits Inflammation by Modulating the NF-κB and Nrf2/HO-1 Signaling Pathways in Macrophage Cells. Antioxidants 2021, 10, 1208. https://doi.org/10.3390/antiox10081208
Kim M, Kim JY, Yang HS, Choe J-S, Hwang IG. Nepetoidin B from Salvia plebeia R. Br. Inhibits Inflammation by Modulating the NF-κB and Nrf2/HO-1 Signaling Pathways in Macrophage Cells. Antioxidants. 2021; 10(8):1208. https://doi.org/10.3390/antiox10081208
Chicago/Turabian StyleKim, Mina, Ji Yeong Kim, Hee Sun Yang, Jeong-Sook Choe, and In Guk Hwang. 2021. "Nepetoidin B from Salvia plebeia R. Br. Inhibits Inflammation by Modulating the NF-κB and Nrf2/HO-1 Signaling Pathways in Macrophage Cells" Antioxidants 10, no. 8: 1208. https://doi.org/10.3390/antiox10081208