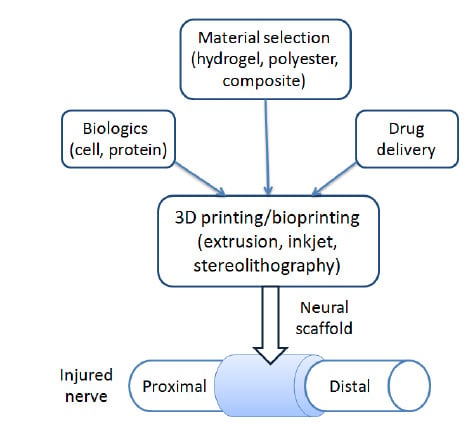
3D Printing and Bioprinting Nerve Conduits for Neural Tissue Engineering
Abstract
:1. Introduction
2. Requirements for Ideal Peripheral Neural Scaffolds
3. 3D Printing Technologies for Nerve Regeneration
3.1. Basic 3D Printing Technologies
3.2. Emerging 3D Printing Technologies
4. 3D Printing Materials for Nerve Regeneration
4.1. Hydrogels
4.1.1. Printable Hydrogels
4.1.2. Natural Hydrogels
4.1.3. Synthetic Hydrogels
4.1.4. Composite Hydrogels
4.2. Polyesters
4.3. Composites
4.3.1. Conductive Polymers
4.3.2. Carbon-Based Nanomaterials
5. 3D Bioprinting and Therapeutics Delivery for Nerve Regeneration
5.1. Bioprinting Methods
5.2. Cells and Bioactive Molecules
5.3. Therapeutics Delivery
6. Summary and Prospect
Funding
Conflicts of Interest
References
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J.; Reid, A.J. Peripheral nerve regeneration: Experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015, 82, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandran, V.; Coppola, G.; Nawabi, H.; Omura, T.; Versano, R.; Huebner, E.A.; Zhang, A.; Costigan, M.; Yekkirala, A.; Barrett, L.; et al. A Systems-Level Analysis of the Peripheral Nerve Intrinsic Axonal Growth Program. Neuron 2016, 89, 956–970. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Fontan, F.; Reeves, B.; Tuaño, K.; Colakoglu, S.; D’Agostino, L.; Banegas, R. Tobacco use and neurogenesis: A theoretical review of pathophysiological mechanism affecting the outcome of peripheral nerve regeneration. J. Orthop. 2020, 22, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Houdek, M.T.; Shin, A.Y. Management and Complications of Traumatic Peripheral Nerve Injuries. Hand Clin. 2015, 31, 151–163. [Google Scholar] [CrossRef]
- Belkas, J.; Shoichet, M.; Midha, R. Peripheral Nerve Regeneration Through Guidance Tubes. Neurol. Res. 2004, 26, 151–160. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and Engineering the Cell Surface Interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef]
- Kornfeld, T.; Vogt, P.M.; Radtke, C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien. Med. Wochenschr. 2019, 169, 240–251. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Liaw, C.-Y.; Guvendiren, M. Current and emerging applications of 3D printing in medicine. Biofabrication 2017, 9, 24102. [Google Scholar] [CrossRef] [PubMed]
- Jakus, A.E.; Rutz, A.L.; Shah, R.N. Advancing the field of 3D biomaterial printing. Biomed. Mater. 2016, 11, 14102. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.R.; Thakur, T.; Desai, P.; Jaiswal, M.K.; Sears, N.; Cosgriff-Hernandez, E.; Kaunas, R.; Gaharwar, A.K. Bioactive Nanoengineered Hydrogels for Bone Tissue Engineering: A Growth-Factor-Free Approach. ACS Nano 2015, 9, 3109–3118. [Google Scholar] [CrossRef] [PubMed]
- Mosadegh, B.; Xiong, G.; Dunham, S.; Min, J. Current progress in 3D printing for cardiovascular tissue engineering. Biomed. Mater. 2015, 10, 034002. [Google Scholar] [CrossRef]
- Hsieh, F.-Y.; Lin, H.-H.; Hsu, S. 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 2015, 71, 48–57. [Google Scholar] [CrossRef]
- Hur, J.; Im, K.; Kim, S.W.; Kim, J.; Chung, D.-Y.; Kim, T.-H.; Jo, K.H.; Hahn, J.H.; Bao, Z.; Hwang, S.; et al. Polypyrrole/Agarose-Based Electronically Conductive and Reversibly Restorable Hydrogel. ACS Nano 2014, 8, 10066–10076. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Jun, Y.-J.; Kim, D.Y.; Yi, H.-G.; Chae, S.-H.; Kang, J.; Lee, J.; Gao, G.; Kong, J.-S.; Jang, J.; et al. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials 2019, 206, 160–169. [Google Scholar] [CrossRef]
- Mobaraki, M.; Ghaffari, M.; Yazdanpanah, A.; Luo, Y.; Mills, D.K. Bioinks and bioprinting: A focused review. Bioprinting 2020, 18, e00080. [Google Scholar] [CrossRef]
- Alluin, O.; Wittmann, C.; Marqueste, T.; Chabas, J.-F.; Garcia, S.; Lavaut, M.-N.; Guinard, D.; Feron, F.; Decherchi, P. Functional recovery after peripheral nerve injury and implantation of a collagen guide. Biomaterials 2009, 30, 363–373. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Yucel, D.; Kose, G.T.; Hasirci, V. Polyester based nerve guidance conduit design. Biomaterials 2010, 31, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2019. [Google Scholar] [CrossRef]
- Tamay, D.G.; Dursun Usal, T.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for neural tissue engineering applications. Mater. Today Bio. 2020, 6, 100043. [Google Scholar] [CrossRef] [PubMed]
- Place, E.; Evans, N.; Stevens, M. Complexity in Biomaterials for Tissue Engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- Dixon, A.R.; Jariwala, S.H.; Bilis, Z.; Loverde, J.R.; Pasquina, P.F.; Alvarez, L.M. Bridging the gap in peripheral nerve repair with 3D printed and bioprinted conduits. Biomaterials 2018, 186, 44–63. [Google Scholar] [CrossRef]
- Zhu, W.; Tringale, K.R.; Woller, S.A.; You, S.; Johnson, S.; Shen, H.; Schimelman, J.; Whitney, M.; Steinauer, J.; Xu, W.; et al. Rapid continuous 3D printing of customizable peripheral nerve guidance conduits. Mater. Today 2018, 21, 951–959. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Hu, T.; Ma, P.X.; Guo, B. Aligned conductive core-shell biomimetic scaffolds based on nanofiber yarns/hydrogel for enhanced 3D neurite outgrowth alignment and elongation. Acta Biomater. 2019, 96, 175–187. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Oliveira, J.M.; Reis, R.L. Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit. Front. Bioeng. Biotechnol. 2019, 7, 337. [Google Scholar] [CrossRef] [Green Version]
- Borschel, G.H.; Kia, K.F.; Kuzon, W.M.; Dennis, R.G. Mechanical properties of acellular peripheral nerve. J. Surg. Res. 2003, 114, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J. Biomed. Sci. 2009, 16, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-J.; Zhu, W.; Castro, N.; Zhang, L.G. Biomaterials and 3D Printing Techniques for Neural Tissue Regeneration. In Neural Engineering: From Advanced Biomaterials to 3D Fabrication Techniques; Zhang, L.G., Kaplan, D.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–24. [Google Scholar]
- Dermanaki Farahani, R.; Dubé, M. Printing Polymer Nanocomposites and Composites in Three Dimensions. Adv. Eng. Mater. 2018, 20, 1–9. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lee, J.; Park, S.A.; Kim, W.D. Three-dimensional bio-printing equipment technologies for tissue engineering and regenerative medicine. Tissue Eng. Regen. Med. 2016, 13, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of inkjet printing to tissue engineering. Biotechnol. J. 2006, 1, 910–917. [Google Scholar] [CrossRef]
- Radulescu, D.; Dhar, S.; Young, C.M.; Taylor, D.W.; Trost, H.-J.; Hayes, D.J.; Evans, G.R. Tissue engineering scaffolds for nerve regeneration manufactured by ink-jet technology. Mater. Sci. Eng. C 2007, 27, 534–539. [Google Scholar] [CrossRef]
- Hsiao, D.; Hsu, S.-H.; Chen, R.-S.; Chen, M.-H. Characterization of designed directional polylactic acid 3D scaffolds for neural differentiation of human dental pulp stem cells. J. Formos. Med. Assoc. 2020, 119, 268–275. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [Green Version]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef] [PubMed]
- Hornbeck, L.J. Deformable-Mirror Spatial Light Modulators. In Proceedings of SPIE; SPIE: San Diego, CA, USA, 1990; Volume 1150. [Google Scholar]
- Ye, W.; Li, H.; Yu, K.; Xie, C.; Wang, P.; Zheng, Y.; Zhang, P.; Xiu, J.; Yang, Y.; Zhang, F.; et al. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater. Des. 2020, 192, 108757. [Google Scholar] [CrossRef]
- Maruo, S.; Nakamura, O.; Kawata, S. Three-dimensional microfabrication with two-photon-absorbed photopolymerization. Opt. Lett. 1997, 22, 132–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawata, S.; Sun, H.-B.; Tanaka, T.; Takada, K. Finer features for functional microdevices. Nature 2001, 412, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Kim, R.H.; Yang, D.-Y.; Park, S.H. Advances in 3D nano/microfabrication using two-photon initiated polymerization. Prog. Polym. Sci. 2008, 33, 631–681. [Google Scholar] [CrossRef]
- Liska, R.; Schuster, M.; Inführ, R.; Turecek, C.; Fritscher, C.; Seidl, B.; Schmidt, V.; Kuna, L.; Haase, A.; Varga, F.; et al. Photopolymers for rapid prototyping. J. Coat. Technol. Res. 2007, 4, 505–510. [Google Scholar] [CrossRef]
- Xing, J.-F.; Dong, X.-Z.; Chen, W.-Q.; Duan, X.-M.; Takeyasu, N.; Tanaka, T.; Kawata, S. Improving spatial resolution of two-photon microfabrication by using photoinitiator with high initiating efficiency. Appl. Phys. Lett. 2007, 90, 131106. [Google Scholar] [CrossRef]
- Koroleva, A.; Gill, A.; Ortega, I.; Haycock, J.; Schlie, S.; Gittard, S.; Chichkov, B.; Claeyssens, F. Two-photon polymerization-generated and micromolding-replicated 3D scaffolds for peripheral neural tissue engineering applications. Biofabrication 2012, 4, 25005. [Google Scholar] [CrossRef]
- Accardo, A.; Blatche, M.-C.; Courson, R.; Loubinoux, I.; Vieu, C.; Malaquin, L. Two-photon lithography and microscopy of 3D hydrogel scaffolds for neuronal cell growth. Biomed. Phys. Eng. Express 2018, 4, 027009. [Google Scholar] [CrossRef]
- Marino, A.; Ciofani, G.; Filippeschi, C.; Pellegrino, M.; Pellegrini, M.; Orsini, P.; Pasqualetti, M.; Mattoli, V.; Mazzolai, B. Two-Photon Polymerization of Sub-micrometric Patterned Surfaces: Investigation of Cell-Substrate Interactions and Improved Differentiation of Neuron-like Cells. ACS Appl. Mater. Interfaces 2013, 5, 13012–13021. [Google Scholar] [CrossRef]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A.; et al. Continuous liquid interface production of 3D objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Bloomquist, C.J.; Mecham, M.B.; Paradzinsky, M.D.; Janusziewicz, R.; Warner, S.B.; Luft, J.C.; Mecham, S.J.; Wang, A.Z.; DeSimone, J.M. Controlling release from 3D printed medical devices using CLIP and drug-loaded liquid resins. J. Control. Release 2018, 278, 9–23. [Google Scholar] [CrossRef]
- Li, H.; Tan, C.; Li, L. Review of 3D printable hydrogels and constructs. Mater. Des. 2018, 159, 20–38. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef]
- Nadernezhad, A.; Khani, N.; Skvortsov, G.A.; Toprakhisar, B.; Bakirci, E.; Menceloglu, Y.; Unal, S.; Koc, B. Multifunctional 3D printing of heterogeneous hydrogel structures. Sci. Rep. 2016, 6, 33178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stauffer, S.R.; Peppast, N.A. Poly(vinyl alcohol) hydrogels prepared by freezing-thawing cyclic processing. Polymer (Guildf) 1992, 33, 3932–3936. [Google Scholar] [CrossRef]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Yu, X.; Long, Q.; Zhang, T. Synthesis of a photocurable acrylated poly(ethylene glycol)-co-poly(xylitol sebacate) copolymers hydrogel 3D printing ink for tissue engineering. RSC Adv. 2019, 9, 18394–18405. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, C.; Luo, X.; Wang, X.; Jiang, H. Recent advances of collagen-based biomaterials: Multi-hierarchical structure, modification and biomedical applications. Mater. Sci. Eng. C 2019, 99, 1509–1522. [Google Scholar] [CrossRef]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Aregueta-Robles, U.A.; Martens, P.J.; Poole-Warren, L.A.; Green, R.A. Tissue engineered hydrogels supporting 3D neural networks. Acta Biomater. 2019, 95, 269–284. [Google Scholar] [CrossRef]
- Kamaci, M. Polyurethane-based hydrogels for controlled drug delivery applications. Eur. Polym. J. 2020, 123, 109444. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorobieva, E.; Basalyga, I.; Krutko, N. Colloid-chemical properties of polymeric complexes based on polycarboxylic acids and polyacrylamide. Mater. Res. Innov. 2003, 7, 322–325. [Google Scholar] [CrossRef]
- Ciardelli, G.; Chiono, V. Materials for peripheral nerve regeneration. Macromol. Biosci. 2006, 6, 13–26. [Google Scholar] [CrossRef]
- Agulhon, P.; Robitzer, M.; Habas, J.-P.; Quignard, F. Influence of both cation and alginate nature on the rheological behavior of transition metal alginate gels. Carbohydr. Polym. 2014, 112, 525–531. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, T.; Suzuki, Y.; Kitada, M.; Kataoka, K.; Wu, S.; Suzuki, K.; Endo, K.; Nishimura, Y.; Ide, C. Peripheral nerve regeneration through alginate gel: Analysis of early outgrowth and late increase in diameter of regenerating axons. Exp. Brain Res. 2002, 146, 356–368. [Google Scholar] [CrossRef]
- Naghieh, S.; Sarker, M.D.; Abelseth, E.; Chen, X. Indirect 3D bioprinting and characterization of alginate scaffolds for potential nerve tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2019, 93, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Li, G.; Xue, C.; Wang, H.; Yang, X.; Zhao, Y.; Zhang, L.; Yang, Y. Spatially featured porous chitosan conduits with micropatterned inner wall and seamless sidewall for bridging peripheral nerve regeneration. Carbohydr. Polym. 2018, 194, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Therriault, D.; Heuzey, M.-C. Processing and Properties of Chitosan Inks for 3D Printing of Hydrogel Microstructures. ACS Biomater. Sci. Eng. 2018, 4, 2643–2652. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Wrobel, S.; Meyer, C.; Brandenberger, C.; Cengiz, I.F.; López-Cebral, R.; Silva-Correia, J.; Ronchi, G.; Reis, R.L.; Grothe, C.; et al. Gellan Gum-based luminal fillers for peripheral nerve regeneration: An in vivo study in the rat sciatic nerve repair model. Biomater. Sci. 2018, 6, 1059–1075. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Zhang, S. Long-term three-dimensional neural tissue cultures in functionalized self-assembling peptide hydrogels, Matrigel and Collagen I. Acta Biomater. 2013, 9, 5162–5169. [Google Scholar] [CrossRef]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef]
- Bozkurt, A.; Boecker, A.; Tank, J.; Altinova, H.; Deumens, R.; Dabhi, C.; Tolba, R.; Weis, J.; Brook, G.A.; Pallua, N.; et al. Efficient bridging of 20 mm rat sciatic nerve lesions with a longitudinally micro-structured collagen scaffold. Biomaterials 2016, 75, 112–122. [Google Scholar] [CrossRef]
- Tao, J.; Hu, Y.; Wang, S.; Zhang, J.; Liu, X.; Gou, Z.; Cheng, H.; Liu, Q.; Zhang, Q.; You, S.; et al. A 3D-engineered porous conduit for peripheral nerve repair. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef]
- Zhang, J.; Allardyce, B.J.; Rajkhowa, R.; Zhao, Y.; Dilley, R.J.; Redmond, S.L.; Wang, X.; Liu, X. 3D Printing of Silk Particle-Reinforced Chitosan Hydrogel Structures and Their Properties. ACS Biomater. Sci. Eng. 2018, 4, 3036–3046. [Google Scholar] [CrossRef]
- Jiang, J.-P.; Liu, X.-Y.; Zhao, F.; Zhu, X.; Li, X.-Y.; Niu, X.-G.; Yao, Z.-T.; Dai, C.; Xu, H.-Y.; Ma, K.; et al. Three-dimensional bioprinting collagen/silk fibroin scaffold combined with neural stem cells promotes nerve regeneration after spinal cord injury. Neural Regen. Res. 2020, 15, 959–968. [Google Scholar]
- Kuzmenko, V.; Karabulut, E.; Pernevik, E.; Enoksson, P.; Gatenholm, P. Tailor-made conductive inks from cellulose nanofibrils for 3D printing of neural guidelines. Carbohydr. Polym. 2018, 189, 22–30. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Q.; Xie, S.; Shan, X.; Cai, Z. In vitro and in vivo biocompatibility evaluation of a 3D bioprinted gelatin-sodium alginate/rat Schwann-cell scaffold. Mater. Sci. Eng. C 2020, 109, 110530. [Google Scholar] [CrossRef]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H.; et al. Structurally and Functionally Optimized Silk-Fibroin–Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Sochilina, A.V.; Budylin, N.Y.; Gamisonia, A.M.; Chalykh, A.E.; Zubov, V.P.; Vikhrov, A.A. Multichannel hydrogel based on a chitosan–poly(vinyl alcohol) composition for directed growth of animal cells. Colloids Surf. B Biointerfaces 2019, 184, 110495. [Google Scholar] [CrossRef]
- Meng, Y.; Cao, J.; Chen, Y.; Yu, Y.; Ye, L. 3D printing of a poly(vinyl alcohol)-based nano-composite hydrogel as an artificial cartilage replacement and the improvement mechanism of printing accuracy. J. Mater. Chem. B 2020, 8, 677–690. [Google Scholar] [CrossRef]
- Ho, L.; Hsu, S. Cell reprogramming by 3D bioprinting of human fibroblasts in polyurethane hydrogel for fabrication of neural-like constructs. Acta Biomater. 2018, 70, 57–70. [Google Scholar] [CrossRef]
- Huang, C.-T.; Shrestha, L.; Ariga, K.; Hsu, S. A graphene–polyurethane composite hydrogel as a potential bioink for 3D bioprinting and differentiation of neural stem cells. J. Mater. Chem. B 2017, 5, 8854–8864. [Google Scholar] [CrossRef]
- Pateman, C.J.; Harding, A.J.; Glen, A.; Taylor, C.S.; Christmas, C.R.; Robinson, P.P.; Rimmer, S.; Boissonade, F.M.; Claeyssens, F.; Haycock, J.W. Nerve guides manufactured from photocurable polymers to aid peripheral nerve repair. Biomaterials 2015, 49, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Dilla, R.A.; Motta, C.M.; Snyder, S.R.; Wilson, J.A.; Wesdemiotis, C.; Becker, M.L. Synthesis and 3D Printing of PEG-Poly(propylene fumarate) Diblock and Triblock Copolymer Hydrogels. ACS Macro Lett. 2018, 7, 1254–1260. [Google Scholar] [CrossRef]
- Raza, C.; Riaz, H.A.; Anjum, R.; Shakeel, N. Repair strategies for injured peripheral nerve: Review. Life Sci. 2020, 243, 117308. [Google Scholar] [CrossRef]
- Lendlein, A.; Trask, R.S. Multifunctional materials: Concepts, function-structure relationships, knowledge-based design, translational materials research. Multifunct. Mater. 2018, 1, 10201. [Google Scholar] [CrossRef]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef]
- Buwalda, S.J. Bio-based composite hydrogels for biomedical applications. Multifunct. Mater. 2020, 3, 22001. [Google Scholar] [CrossRef]
- Lee, Y.-B.; Polio, S.; Lee, W.; Dai, G.; Menon, L.; Carroll, R.S.; Yoo, S.-S. Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp. Neurol. 2010, 223, 645–652. [Google Scholar] [CrossRef]
- Jafarkhani, M.; Salehi, Z.; Nematian, T. Preparation and characterization of chitosan/graphene oxide composite hydrogels for nerve tissue Engineering. Mater. Today Proc. 2018, 5, 15620–15628. [Google Scholar] [CrossRef]
- Chen, J.; Huang, D.; Wang, L.; Hou, J.; Zhang, H.; Li, Y.; Zhong, S.; Wang, Y.; Wu, Y.; Huang, W. 3D bioprinted multiscale composite scaffolds based on gelatin methacryloyl (GelMA)/chitosan microspheres as a modular bioink for enhancing 3D neurite outgrowth and elongation. J. Colloid Interface Sci. 2020, 574, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Zhu, W.; Heyburn, L.; Nowicki, M.; Harris, B.; Zhang, L.G. Development of Novel 3-D Printed Scaffolds With Core-Shell Nanoparticles for Nerve Regeneration. IEEE Trans. Biomed. Eng. 2017, 64, 408–418. [Google Scholar] [CrossRef]
- Arslantunali, D.; Dursun, T.; Yucel, D.; Hasirci, N.; Hasirci, V. Peripheral nerve conduits: Technology update. Med. Devices (Auckl. NZ) 2014, 7, 405–424. [Google Scholar]
- Jahromi, H.K.; Farzin, A.; Hasanzadeh, E.; Barough, S.E.; Mahmoodi, N.; Najafabadi, M.R.H.; Farahani, M.S.; Mansoori, K.; Shirian, S.; Ai, J. Enhanced sciatic nerve regeneration by poly-L-lactic acid/multi-wall carbon nanotube neural guidance conduit containing Schwann cells and curcumin encapsulated chitosan nanoparticles in rat. Mater. Sci. Eng. C 2020, 109, 110564. [Google Scholar] [CrossRef]
- Freed, L.E.; Vunjak-Novakovic, G.; Biron, R.J.; Eagles, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable Polymer Scaffolds for Tissue Engineering. Bio/Technology 1994, 12, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Molde, J.; Soares, R.M.D.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F. Biodegradable Polymers for Biomedical Additive Manufacturing. Appl. Mater. Today 2020, 20, 100700. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Zhang, S.; Thaharah, S.; Sriram, G.; Lu, W.F.; Fuh, J.Y.H. Electrohydrodynamic Jet 3D Printed Nerve Guide Conduits (NGCs) for Peripheral Nerve Injury Repair. Polymers (Basel) 2018, 10, 753. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Peng, X.; Nelson, K.D.; Eberhart, R.; Smith, G.M. Permeable guidance channels containing microfilament scaffolds enhance axon growth and maturation. J. Biomed. Mater. Res. Part A 2005, 75, 374–386. [Google Scholar] [CrossRef]
- Frattini, F.; Pereira Lopes, F.R.; Almeida, F.M.; Rodrigues, R.F.; Boldrini, L.C.; Tomaz, M.A.; Baptista, A.F.; Melo, P.A.; Martinez, A.M.B. Mesenchymal Stem Cells in a Polycaprolactone Conduit Promote Sciatic Nerve Regeneration and Sensory Neuron Survival after Nerve Injury. Tissue Eng. Part A 2012, 18, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhao, X.; Han, Q.; Chen, W.; Li, H.; Yuan, W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Thaharah, S.; Zhang, S.; Lu, W.F.; Fuh, J.Y.H. Electrohydrodynamic jet 3D-printed PCL/PAA conductive scaffolds with tunable biodegradability as nerve guide conduits (NGCs) for peripheral nerve injury repair. Mater. Des. 2019, 162, 171–184. [Google Scholar] [CrossRef]
- Wang, X.; Cui, T.; Yan, Y.; Zhang, R. Peroneal nerve regeneration using a unique bilayer polyurethane-collagen guide conduit. J. Bioact. Compat. Polym. 2009, 24, 109–127. [Google Scholar] [CrossRef]
- Kaplan, B.; Merdler, U.; Szklannya, A.A.; Redenski, I.; Guo, S.; Bar-Mucha, Z.; Michael, N.; Levenberg, S. Rapid prototyping fabrication of soft and oriented polyester scaffolds for axonal guidance. Biomaterials 2020, 251, 120062. [Google Scholar] [CrossRef]
- Hassan, M.; Dave, K.; Chandrawati, R.; Dehghani, F.; Gomes, V.G. 3D printing of biopolymer nanocomposites for tissue engineering: Nanomaterials, processing and structure-function relation. Eur. Polym. J. 2019, 121, 109340. [Google Scholar] [CrossRef]
- Zhu, W.; Ye, T.; Lee, S.-J.; Cui, H.; Miao, S.; Zhou, X.; Shuai, D.; Zhang, L.G. Enhanced neural stem cell functions in conductive annealed carbon nanofibrous scaffolds with electrical stimulation. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2485–2494. [Google Scholar] [CrossRef]
- Pires, F.; Ferreira, Q.; Rodrigues, C.A.V.; Morgado, J.; Ferreira, F.C. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1158–1168. [Google Scholar] [CrossRef]
- Song, S.; Amores, D.; Chen, C.; McConnell, K.; Oh, B.; Poon, A.; George, P.M. Controlling properties of human neural progenitor cells using 2D and 3D conductive polymer scaffolds. Sci. Rep. 2019, 9, 19565. [Google Scholar] [CrossRef]
- Wu, C.; Liu, A.; Chen, S.; Zhang, X.; Chen, L.; Zhu, Y.; Xiao, Z.; Sun, J.; Luo, H.; Fan, H. Cell-Laden Electroconductive Hydrogel Simulating Nerve Matrix to Deliver Electrical Cues and Promote Neurogenesis. ACS Appl. Mater. Interfaces 2019, 11, 22152–22163. [Google Scholar] [CrossRef]
- Alizadeh, R.; Zarrintaj, P.; Kamrava, S.K.; Bagher, Z.; Farhadi, M.; Heidari, F.; Komeili, A.; Gutiérrez, T.J.; Saeb, M.R. Conductive hydrogels based on agarose/alginate/chitosan for neural disorder therapy. Carbohydr. Polym. 2019, 224, 115161. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Jia, R.; Gan, Z.; Du, Y.; Wang, D.; Xu, X. Tough and conductive polymer hydrogel based on double network for photo-curing 3D printing. Mater. Res. Express 2020, 7, 55304. [Google Scholar] [CrossRef]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef] [Green Version]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Heo, D.N.; Lee, S.-J.; Timsina, R.; Qiu, X.; Castro, N.J.; Zhang, L.G. Development of 3D printable conductive hydrogel with crystallized PEDOT:PSS for neural tissue engineering. Mater. Sci. Eng. C 2019, 99, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Fantino, E.; Roppolo, I.; Zhang, D.; Xiao, J.; Chiappone, A.; Castellino, M.; Guo, Q.; Pirri, C.F.; Yang, J. 3D Printing/Interfacial Polymerization Coupling for the Fabrication of Conductive Hydrogel. Macromol. Mater. Eng. 2018, 303, 1700356. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Kannan, S.; Cao, T.; Fuh, J.Y.H.; Sriram, G.; Lu, W.F. 3D-Printed PCL/PPy Conductive Scaffolds as Three-Dimensional Porous Nerve Guide Conduits (NGCs) for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2019, 7, 266. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon N. Y. 2010, 48, 2127–2150. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Fraczek-Szczypta, A. Carbon nanomaterials for nerve tissue stimulation and regeneration. Mater. Sci. Eng. C 2014, 34, 35–49. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, J.; Sim, S.H.; Sung, M.G.; Kim, K.S.; Hong, B.H.; Hong, S. Enhanced Differentiation of Human Neural Stem Cells into Neurons on Graphene. Adv. Mater. 2011, 23, H263–H267. [Google Scholar] [CrossRef]
- Tran, P.A.; Zhang, L.; Webster, T.J. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1097–1114. [Google Scholar] [CrossRef]
- Uz, M.; Donta, M.; Mededovic, M.; Sakaguchi, D.; Mallapragada, S. Development of Gelatin and Graphene-Based Nerve Regeneration Conduits Using 3D Printing Strategies for Electrical Transdifferentiation of Mesenchymal Stem Cells. Ind. Eng. Chem. Res. 2019, 58, 7421–7427. [Google Scholar] [CrossRef] [Green Version]
- Koppes, A.N.; Keating, K.W.; McGregor, A.L.; Koppes, R.A.; Kearns, K.R.; Ziemba, A.M.; McKay, C.A.; Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; et al. Robust neurite extension following exogenous electrical stimulation within single walled carbon nanotube-composite hydrogels. Acta Biomater. 2016, 39, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-Dimensional Printing of High-Content Graphene Scaffolds for Electronic and Biomedical Applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Lee, S.-J.; Zhu, W.; Nowicki, M.; Lee, G.; Heo, D.N.; Kim, J.; Zuo, Y.Y.; Zhang, L.G. 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. J. Neural Eng. 2018, 15, 16018. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.; Yeong, W.Y. Bioprinting: Principles and Applications; World Scientific Publishing Company: Hackensack, NJ, USA, 2015. [Google Scholar]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.; Whiteley, R.; Yu, T.; Stringer, J.; Macneil, S.; Haycock, J.; Smith, P. Inkjet printing Schwann cells and neuronal analogue NG108-15 cells. Biofabrication 2016, 8, 15017. [Google Scholar] [CrossRef]
- Koch, L.; Gruene, M.; Chichkov, C.U.; Chichkov, B. Laser Assisted Cell Printing. Curr. Pharm. Biotechnol. 2013, 14, 91–97. [Google Scholar] [PubMed]
- Bedir, T.; Ulag, S.; Ustundag, C.B.; Gunduz, O. 3D bioprinting applications in neural tissue engineering for spinal cord injury repair. Mater. Sci. Eng. C 2020, 110, 110741. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, A.; Schreyer, D.; Chen, D. Bioplotting Alginate/Hyaluronic Acid Hydrogel Scaffolds with Structural Integrity and Preserved Schwann Cell Viability. 3D Print. Addit. Manuf. 2014, 1, 194–203. [Google Scholar] [CrossRef]
- Moldovan, N.I.; Hibino, N.; Nakayama, K. Principles of the Kenzan Method for Robotic Cell Spheroid-Based Three-Dimensional Bioprinting. Tissue Eng. Part B Rev. 2016, 23, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar, I.N.; Olivos, D.J.; Brinker, A.; Alvarez, M.B.; Smith, L.J.; Chu, T.-M.G.; Kacena, M.A.; Wagner, D.R. Scaffold-free bioprinting of mesenchymal stem cells using the Regenova printer: Spheroid characterization and osteogenic differentiation. Bioprinting 2019, 15, e00050. [Google Scholar] [CrossRef]
- Zhang, Q.; Nguyen, P.D.; Shi, S.; Burrell, J.C.; Cullen, D.K.; Le, A.D. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci. Rep. 2018, 8, 6634. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, I.N.; Smith, L.J.; Olivos, D.J.; Chu, T.-M.G.; Kacena, M.A.; Wagner, D.R. Scaffold-free bioprinting of mesenchymal stem cells with the regenova printer: Optimization of printing parameters. Bioprinting 2019, 15, e00048. [Google Scholar] [CrossRef]
- Ong, W.; Pinese, C.; Chew, S.Y. Scaffold-mediated sequential drug/gene delivery to promote nerve regeneration and remyelination following traumatic nerve injuries. Adv. Drug Deliv. Rev. 2019, 149–150, 19–48. [Google Scholar] [CrossRef]
- Accardo, A.; Cirillo, C.; Lionnet, S.; Vieu, C.; Loubinoux, I. Interfacing cells with microengineered scaffolds for neural tissue reconstruction. Brain Res. Bull. 2019, 152, 202–211. [Google Scholar] [CrossRef]
- Namgung, U. The Role of Schwann Cell-Axon Interaction in Peripheral Nerve Regeneration. Cells Tissues Organs 2014, 200, 6–12. [Google Scholar] [CrossRef]
- Panagopoulos, G.; Megaloikonomos, P.; Mavrogenis, A. The Present and Future for Peripheral Nerve Regeneration. Orthopedics 2016, 40, e141–e146. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Barbon, S.; Stocco, E.; Dalzoppo, D.; Contran, M.; Rose, E.; Parnigotto, P.; Macchi, V.; Grandi, C.; Caro, R. Development of Oxidized Polyvinyl Alcohol-Based Nerve Conduits Coupled with the Ciliary Neurotrophic Factor. Materials (Basel) 2019, 12, 1996. [Google Scholar] [CrossRef] [Green Version]
- Sebben, A.D.; Lichtenfels, M.; da Silva, J.L.B. Peripheral Nerve Regeneration: Cell Therapy and Neurotrophic Factors. Rev. Bras. Ortop. (Engl. Ed.) 2011, 46, 643–649. [Google Scholar] [CrossRef]
- Fu, S.Y.; Gordon, T. The cellular and molecular basis of peripheral nerve regeneration. Mol. Neurobiol. 1997, 14, 67–116. [Google Scholar] [CrossRef] [PubMed]
- Ilkhanizadeh, S.; Teixeira, A.I.; Hermanson, O. Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials 2007, 28, 3936–3943. [Google Scholar] [CrossRef] [PubMed]
- Joung, D.; Truong, V.; Neitzke, C.C.; Guo, S.-Z.; Walsh, P.J.; Monat, J.R.; Meng, F.; Park, S.H.; Dutton, J.R.; Parr, A.M.; et al. 3D Printed Stem-Cell Derived Neural Progenitors Generate Spinal Cord Scaffolds. Adv. Funct. Mater. 2018, 28, 1801850. [Google Scholar] [CrossRef]
- Manoukian, O.S.; Arul, M.R.; Rudraiah, S.; Kalajzic, I.; Kumbar, S.G. Aligned microchannel polymer-nanotube composites for peripheral nerve regeneration: Small molecule drug delivery. J. Control. Release 2019, 296, 54–67. [Google Scholar] [CrossRef]
- Tseng, K.-C.; Li, H.; Clark, A.; Sundem, L.; Zuscik, M.; Noble, M.; Elfar, J. 4-Aminopyridine promotes functional recovery and remyelination in acute peripheral nerve injury. EMBO Mol. Med. 2016, 8, 1409–1420. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Tao, J.; Wang, S.; Yang, L.; Zhang, J.; Zhang, J.; Liu, H.; Cheng, H.; Xu, J.; Gou, M.; et al. 3D printing of nerve conduits with nanoparticle-encapsulated RGFP966. Appl. Mater. Today 2019, 16, 247–256. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, J.; Du, T.; Xu, X.; Deng, X.; Chen, S.; Liu, J.; Chen, Y.; Liu, X.; Xiong, M.; et al. Rapid 3D printing of functional nanoparticle-enhanced conduits for effective nerve repair. Acta Biomater. 2019, 90, 49–59. [Google Scholar] [CrossRef]
- Sinha, S.K. Additive manufacturing (AM) of medical devices and scaffolds for tissue engineering based on 3D and 4D printing. In 3D and 4D Printing of Polymer Nanocomposite Materials; Sadasivuni, K.K., Deshmukh, K., Almaadeed, M.A.B.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 5; pp. 119–160. [Google Scholar]
- Zhu, W.; George, J.; Sorger, V.; Zhang, L. 3D printing scaffold coupled with low level light therapy for neural tissue regeneration. Biofabrication 2017, 9, 025002. [Google Scholar] [CrossRef] [PubMed]





| Materials | Advantages | Disadvantages |
|---|---|---|
| Natural hydrogels (alginate hydrogel [59], chitosan hydrogenl [61], collagen hydrogel [62], gelatin hydrogel [63], silk hydrogel [64] etc.) | Low inflammation; wide variety of sources; good biodegradability and biocompatibility | Poor mechanical properties |
| Synthetic hydrogels (PVA hydrogel [65], PU hydrogel [66], PEG hydrogel [67], PAM hydrogel [68] etc.) | Tunable mechanical properties, degradation rate and biocompatibility; good durability | Possible chronic inflammation |
| Composite hydrogels [69] | Combining the characteristics of different materials; Flexible optimization for the processability of bioprinting | Limitations in homogeneous ink preparation |
| Material | Printing Resolution | Printed Structure | Mechanical Properties | Printing Method | Reference |
|---|---|---|---|---|---|
| PU | 250 μm | Grid scaffold of stacking fibers | - | FDM | [16] |
| PU | 410 μm | Grid scaffold of stacking fibers | - | Extrusion | [94] |
| PU | 410 μm | Grid scaffold of stacking fibers | - | Extrusion | [95] |
| PEGDA | 50 μm | guidance Conduits with trenches | Young’s modulus of 470.0 ± 24.3 MPa | SLA | [96] |
| PEG-PPF | 100 μm | Gyroidal scaffold | Young’s modulus of 9.1 ± 0.1 kPa | DLP | [97] |
| Components | Printing Resolution | Printed Structure | Mechanical Properties | Printing Method | Reference |
|---|---|---|---|---|---|
| Collagen-fibroin hydrogel | 500 μm | Double-layered 3D scaffold | - | Inkjet | [102] |
| Gelatin-alginate hydrogel | 160 μm | Scaffold with void channel | - | Extrusion | [88] |
| PEG-PEGDA hydrogel | 300 μm | Grid scaffold of stacking fibers | Young’s modulus of 1.01 ± 0.11 MPa | SLA | [105] |
| GelMA-PEGDA hydrogel | 2.5 μm | Guidance conduit with four microchannels | Young’s modulus of 0.3~4.5 MPa | DLP | [28] |
| GelMA/GC-MS hydrogel | ~545 ± 61μm | Grid scaffold of stacking fibers | - | Extrusion | [104] |
| Materials | Printing Resolution | Printed Structure | Mechanical Properties | Printing Method | Application | Reference |
|---|---|---|---|---|---|---|
| PLA | 250 μm | Scaffolds with different gap width between struts | - | Extrusion | Promotes neural differentiation of hDPSCs. | [39] |
| PCL | 50 μm | Porous guidance conduit | Elastic modulus of 68.74 MPa | Inkjet | Promotes successful axonal regrowth and remyelination | [114] |
| PLA/PCL | 1 μm | Guidance conduit | - | Inkjet | ability to sustain cell growth and attachment | [38] |
| PCL/PAA | ~50 μm | Grid porous conduit | Young’s modulus of 85 ± 3.9~204 ± 6.7 MPa | EHD jet | Influence nerve excitation and conduction | [115] |
| PU/collagen | ~150 μm | Double layer porous conduit | - | Extrusion | Bridge a 10 mm long rat peroneal nerve defect | [116] |
| PLGA/PLLA | - | Scaffold with guidance channels | Young’s modulus of 2~62 MPa | FDM | Guide axons to linear conformations and support growth of iPSC-derived neurons | [117] |
| Conductive Materials | Main Materials | Printing Resolution (μm) | Printed Structure | Mechanical Property (MPa) | Conductivity (mS/m) | Printing Method | Reference |
|---|---|---|---|---|---|---|---|
| PEDOT | GelMA and PEGDA hydrogel | 200 | Grid scaffold of stacking fibers | Compression stiffness 26.3–35.4 | 1510 | SLA | [129] |
| PPy | PEGDA hydrogel | 200 | Honeycomb structure | Young’s modulus 1.4 | 7.7 | SLA | [130] |
| Graphene | Gelatin hydrogel/PLA | ~100 | Porous conduit | Young’s modulus~80 | 0.02 | Extrusion | [137] |
| Graphene | PLGA | 100 | Square pore scaffolds | Young’s modulus 3~16 | Extrusion | [139] | |
| MWCNT | PEGDA hydrogel | ~200 | Square pore scaffolds | Young’s modulus ~1.1 | ~0.08 | SLA | [140] |
| Graphene | PCL | 50 | Porous conduit | Elastic modulus 68.74 | 890 | Inkjet | [114] |
| Cell | Bioactive Molecule | Material | Printing Resolution | Cell Density | Cell Viability | Printing Method | Application | Reference |
|---|---|---|---|---|---|---|---|---|
| Schwann cells | - | Alginate/HA | 353 ± 7 μm | 5.2 × 105 cells/mL | 92.3% | Bioplotting | Good structural integrity and long-term cell viability | [146] |
| PC12 cell; RSC96 cell | NGF | GelMA/chitosan | 545 ± 61 μm | - | 97.1 ± 3.69% | Bioplotting | Enhance 3D neurite outgrowth and elongation | [104] |
| Schwann cells; NG108-15 cells | - | - | 60 μm | 2 × 105 cells/mL | 89–92%; 86–96% | Inkjet | Neurite outgrows faster and earlier | [143] |
| Schwann cells | - | Gelatin/ sodium alginate | 160 μm | 2 × 106 cells/mL | 91.87 ± 0.55% | Extrusion | Improve cell adhesion and related factor expression | [88] |
| NSCs | - | PU hydrogel | 250 μm | 4 × 106 cells/mL | ~80% | FDM | Promote the recovery of traumatic brain injury in zebrafish | [16] |
| NSCs | VEGF | Collagen hydrogel/fibroin gel | 700 μm | 1 × 106 cells/mL | 93.23 ± 3.77% | Inkjet | Support cellular proliferation and migration over time | [102] |
| Human fibroblasts | Forkhead box D3 | PU hydrogel | 410 μm | 1 × 106 cells/mL | 65% | Extrusion | Human fibroblasts could be reprogrammed into neural crest stem-like cells | [94] |
| NSCs | - | Graphene/PU hydrogel | 410 μm | 4 × 106 cells/mL | >60% | Extrusion | NSCs had a tendency to differentiate toward glial and neuronal lineages | [95] |
| iPSCs | - | GelMA/gelatin/fibroin gel | 200 μm | 1 × 107 cells/mL | >75% | Extrusion | Differentiate and extend axons throughout microscale scaffold channels | [159] |
| GMSCs | - | - | 400~500 µm | - | ~90% | Kenzan method | Promote rat facial nerve regeneration | [149] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Zhang, T.; Li, Y. 3D Printing and Bioprinting Nerve Conduits for Neural Tissue Engineering. Polymers 2020, 12, 1637. https://doi.org/10.3390/polym12081637
Yu X, Zhang T, Li Y. 3D Printing and Bioprinting Nerve Conduits for Neural Tissue Engineering. Polymers. 2020; 12(8):1637. https://doi.org/10.3390/polym12081637
Chicago/Turabian StyleYu, Xiaoling, Tian Zhang, and Yuan Li. 2020. "3D Printing and Bioprinting Nerve Conduits for Neural Tissue Engineering" Polymers 12, no. 8: 1637. https://doi.org/10.3390/polym12081637