Reduction of Biofouling of a Microfiltration Membrane Using Amide Functionalities—Hydrophilization without Changes in Morphology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. PES Membrane Preparation
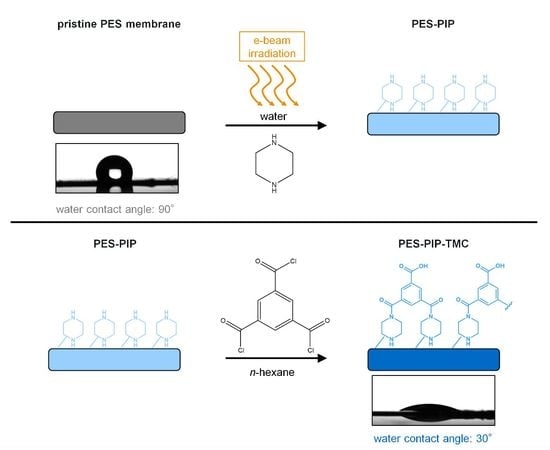
2.3. Membrane Modification
2.4. Membrane Characterization
2.5. Preparation of Fouling Solutions
2.6. Fouling Test
3. Results and Discussion
3.1. Membrane Modification with Amide Fuctionalities
3.2. Algae Fouling
3.3. Stability of the Amide Modification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haynes, C.A.; Norde, W. Structures and Stabilities of Adsorbed Proteins. J. Colloid Interface Sci. 1995, 169, 313–328. [Google Scholar] [CrossRef]
- Wahlgren, M. Protein adsorption to solid surfaces. Trends Biotechnol. 1991, 9, 201–208. [Google Scholar] [CrossRef]
- Trzaskus, K.W.; Wiebe, A.K.; Nijmeijer, K. Towards controlled fouling and rejection in dead-end microfiltration of nanoparticles—Role of electrostatic interactions. J. Membr. Sci. 2015, 496, 174–184. [Google Scholar]
- Lundström, I. Surface Physics and Biological Phenomena. Phys. Scr. 1983, T4, 5–13. [Google Scholar] [CrossRef]
- Chen, H.; Belfort, G. Surface modification of poly(ether sulfone) ultrafiltration membranes by low-temperature plasma-induced graft polymerization. J. Appl. Polym. Sci. 1999, 72, 1699–1711. [Google Scholar] [CrossRef]
- Van Der Bruggen, B. Chemical modification of polyethersulfone nanofiltration membranes: A review. J. Appl. Polym. Sci. 2009, 114, 630–642. [Google Scholar] [CrossRef]
- Chang, Y.; Ko, C.-Y.; Shih, Y.-J.; Quémener, D.; Deratani, A.; Wei, T.-C.; Wang, D.-M.; Lai, J.-Y. Surface grafting control of PEGylated poly(vinylidene fluoride) antifouling membrane via surface-initiated radical graft copolymerization. J. Membr. Sci. 2009, 345, 160–169. [Google Scholar] [CrossRef]
- Saxena, N.; Prabhavathy, C.; De, S.; Dasgupta, S. Flux enhancement by argon–oxygen plasma treatment of polyethersulfone membranes. Sep. Purif. Technol. 2009, 70, 160–165. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.; Wang, L.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R: Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef] [Green Version]
- Du, J.R.; Peldszus, S.; Huck, P.M.; Feng, X. Modification of poly(vinylidene fluoride) ultrafiltration membranes with poly(vinyl alcohol) for fouling control in drinking water treatment. Water Res. 2009, 43, 4559–4568. [Google Scholar] [CrossRef]
- Khulbea, K.; Feng, C.; Matsuura, T. The art of surface modification of synthetic polymeric membranes. J. Appl. Polym. Sci. 2010, 115, 855–895. [Google Scholar] [CrossRef]
- Liu, S.X.; Kim, J.T.; Kim, S. Effect of Polymer Surface Modification on Polymer–Protein Interaction via Hydrophilic Polymer Grafting. J. Food Sci. 2008, 73, 143–150. [Google Scholar] [CrossRef]
- Xiao, K.; Wang, X.; Huang, X.; Waite, T.D.; Wen, X. Combined effect of membrane and foulant hydrophobicity and surface charge on adsorptive fouling during microfiltration. J. Membr. Sci. 2011, 373, 140–151. [Google Scholar] [CrossRef]
- Breite, D.; Went, M.; Prager, A.; Kuehnert, M.; Schulze, A.; Kühnert, M. Charge Separating Microfiltration Membrane with pH-Dependent Selectivity. Polymers 2018, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Hadidi, M.; Zydney, A.L. Fouling behavior of zwitterionic membranes: Impact of electrostatic and hydrophobic interactions. J. Membr. Sci. 2014, 452, 97–103. [Google Scholar] [CrossRef]
- Zhang, Q.; Vecitis, C.D. Conductive CNT-PVDF membrane for capacitive organic fouling reduction. J. Membr. Sci. 2014, 459, 143–156. [Google Scholar] [CrossRef]
- Dudchenko, A.V.; Rolf, J.; Russell, K.; Duan, W.; Jassby, D. Organic fouling inhibition on electrically conducting carbon nanotube–polyvinyl alcohol composite ultrafiltration membranes. J. Membr. Sci. 2014, 468, 1–10. [Google Scholar] [CrossRef]
- Childress, A.E.; Elimelech, M. Relating Nanofiltration Membrane Performance to Membrane Charge (Electrokinetic) Characteristics. Environ. Sci. Technol. 2000, 34, 3710–3716. [Google Scholar] [CrossRef]
- Hong, S.; Elimelech, M. Chemical and physical aspects of natural organic matter (NOM) fouling of nanofiltration membranes. J. Membr. Sci. 1997, 132, 159–181. [Google Scholar] [CrossRef]
- Yang, X.; Yan, L.; Ma, J.; Bai, Y.; Shao, L. Bioadhesion-inspired surface engineering constructing robust, hydrophilic membranes for highly-efficient wastewater remediation. J. Membr. Sci. 2019, 591, 117353. [Google Scholar] [CrossRef]
- Han, M.; Dong, T.; Hou, D.; Yao, J.; Han, L. Carbon nanotube based Janus composite membrane of oil fouling resistance for direct contact membrane distillation. J. Membr. Sci. 2020, 607, 118078. [Google Scholar] [CrossRef]
- Shi, Q.; Su, Y.; Chen, W.; Peng, J.; Nie, L.; Zhang, L.; Jiang, Z. Grafting short-chain amino acids onto membrane surfaces to resist protein fouling. J. Membr. Sci. 2011, 366, 398–404. [Google Scholar] [CrossRef]
- Kumar, M.; Ulbricht, M. Novel ultrafiltration membranes with adjustable charge density based on sulfonated poly(arylene ether sulfone) block copolymers and their tunable protein separation performance. Polymers 2014, 55, 354–365. [Google Scholar] [CrossRef]
- Asfardjani, K.; Segui, Y.; Aurelle, Y.; Abidine, N. Effect of plasma treatments on wettability of polysulfone and polyetherimide. J. Appl. Polym. Sci. 1991, 43, 271–281. [Google Scholar] [CrossRef]
- Boulares-Pender, A.; Thomas, I.; Prager, A.; Schulze, A. Surface modification of polyamide and poly(vinylidene fluoride) membranes. J. Appl. Polym. Sci. 2012, 128, 322–331. [Google Scholar] [CrossRef]
- Buonomenna, M.; Lopez, L.; Favia, P.; D’Agostino, R.; Gordano, A.; Drioli, E. New PVDF membranes: The effect of plasma surface modification on retention in nanofiltration of aqueous solution containing organic compounds. Water Res. 2007, 41, 4309–4316. [Google Scholar] [CrossRef]
- Khulbe, K.; Matsuura, T. Characterization of PPO membranes by oxygen plasma etching, gas separation and atomic force microscopy. J. Membr. Sci. 2000, 171, 273–284. [Google Scholar] [CrossRef]
- Kull, K.R.; Steen, M.L.; Fisher, E.R. Surface modification with nitrogen-containing plasmas to produce hydrophilic, low-fouling membranes. J. Membr. Sci. 2005, 246, 203–215. [Google Scholar] [CrossRef]
- Vandencasteele, N.; Reniers, F. Plasma-modified polymer surfaces: Characterization using XPS. J. Electron Spectrosc. Relat. Phenom. 2010, 178, 394–408. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Chang, C.-Y.; Chien, C.-W.; Huang, L.-K.; Huang, C. Cyclonic plasma surface hydrophilization of polysulfone membrane material. Jpn. J. Appl. Phys. 2019, 59, SAAB01. [Google Scholar] [CrossRef]
- Dvořáková, H.; Čech, J.; Stupavská, M.; Prokeš, L.; Jurmanová, J.; Buršíková, V.; Ráhel’, J.; Ahel, S.; Cech, J.; St’Ahel, P. Fast Surface Hydrophilization via Atmospheric Pressure Plasma Polymerization for Biological and Technical Applications. Polymers 2019, 11, 1613. [Google Scholar] [CrossRef] [Green Version]
- Breite, D.; Went, M.; Prager, A.; Schulze, A. Tailoring Membrane Surface Charges: A Novel Study on Electrostatic Interactions during Membrane Fouling. Polymers 2015, 7, 2017–2030. [Google Scholar] [CrossRef] [Green Version]
- Schulze, A.; Marquardt, B.; Kaczmarek, S.; Schubert, R.; Prager, A.; Buchmeiser, M.R. Electron Beam-Based Functionalization of Poly(ethersulfone) Membranes. Macromol. Rapid Commun. 2010, 31, 467–472. [Google Scholar] [CrossRef]
- Schulze, A.; Marquardt, B.; Went, M.; Prager, A.; Buchmeiser, M.R. Electron beam-based functionalization of polymer membranes. Water Sci. Technol. 2012, 65, 574–580. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hoek, E.M. Impacts of support membrane structure and chemistry on polyamide–polysulfone interfacial composite membranes. J. Membr. Sci. 2009, 336, 140–148. [Google Scholar] [CrossRef]
- Yabuno, Y.; Mihara, K.; Komatsu, K.; Shimamura, S.; Nakagawa, K.; Shintani, T.; Matsuyama, H.; Yoshioka, T. Preparation of Polyamide Thin-Film Composite Membranes Using Hydrophilic Hollow Fiber PVDF via the TIPS Process Modified by PVA Diffusion. Ind. Eng. Chem. Res. 2019, 58, 21691–21699. [Google Scholar] [CrossRef]
- Hurwitz, G.; Guillen, G.R.; Hoek, E.M. Probing polyamide membrane surface charge, zeta potential, wettability, and hydrophilicity with contact angle measurements. J. Membr. Sci. 2010, 349, 349–357. [Google Scholar] [CrossRef]
- Villacorte, L.; Tabatabai, S.; Dhakal, N.; Amy, G.; Schippers, J.; Kennedy, M. Algal blooms: An emerging threat to seawater reverse osmosis desalination. Desalin. Water Treat. 2014, 55, 2601–2611. [Google Scholar] [CrossRef]
- Hung, M.; Liu, J. Microfiltration for separation of green algae from water. Colloids Surf. B Biointerfaces 2006, 51, 157–164. [Google Scholar] [CrossRef]
- Qu, F.; Liang, H.; Wang, Z.; Wang, H.; Yu, H.; Li, G. Ultrafiltration membrane fouling by extracellular organic matters (EOM) of Microcystis aeruginosa in stationary phase: Influences of interfacial characteristics of foulants and fouling mechanisms. Water Res. 2012, 46, 1490–1500. [Google Scholar] [CrossRef]
- Wu, P.; Tang, X.; Liu, Y.; Lin, L.; Xu, C.; Imai, M. An alginate active layer of polyether sulfone membrane suppresses algae-fouling in repeated filtration of Chlorella vulgaris for a higher recovery of water permeation flux. Environ. Sci. Water Res. Technol. 2019, 5, 2162–2171. [Google Scholar] [CrossRef]
- Peng, J.; Su, Y.; Chen, W.; Zhao, X.; Jiang, Z.; Dong, Y.; Zhang, Y.; Liu, J.; Xingzhong, C. Polyamide nanofiltration membrane with high separation performance prepared by EDC/NHS mediated interfacial polymerization. J. Membr. Sci. 2013, 427, 92–100. [Google Scholar] [CrossRef]
- Ghobrini, D.; Potocar, T.; Smolova, J.; Krausova, G.; Yakoub-Bougdal, S.; Branyik, T. Heterotrophic cultivation of Chlorella vulgaris using saline waste water from the demineralization of cheese whey. Biotechnol. Lett. 2019, 42, 209–217. [Google Scholar] [CrossRef]
- Breite, D.; Went, M.; Prager, A.; Schulze, A. The critical zeta potential of polymer membranes: How electrolytes impact membrane fouling. RSC Adv. 2016, 6, 98180–98189. [Google Scholar] [CrossRef]
- Breite, D.; Went, M.; Thomas, I.; Prager, A.; Schulze, A. Particle adsorption on a polyether sulfone membrane: How electrostatic interactions dominate membrane fouling. RSC Adv. 2016, 6, 65383–65391. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]






| Approach | PIP Conc. (30 min) | Electron Beam Dose | TMC Conc. | TMC Application Via | 2nd PIP Conc. (30 min) |
|---|---|---|---|---|---|
| [wt.-%] | [kGy] | [wt.-%] | [wt.-%] | ||
| PES-PA | 0.2 | - | 0.2 | contact on top side for 2 min | - |
| PES-PIP-TMC | 2 | 200 | 0.2 | filtration at 0.5 bar | - |
| PES-PIP-TMC-PIP | 2 | 200 | 0.2 | filtration at 0.5 bar | 0.2 |
| Membrane | Permeance | Porosity | Average Pore Size | Water Contact Angle | Chemical Composition (XPS) [rel. atom-%] | |||
|---|---|---|---|---|---|---|---|---|
| [L (m2 h bar)−1] | [%] | [µm] | [°] | C | O | N | S | |
| REF | 14,600 ± 700 | 72 ± 2 | 0.65 ± 0.04 | 89 ± 5 | 74 ± 2 | 20 ± 2 | - | 5 ± 1 |
| PES-PA | 650 ± 150 | 53 ± 5 | 0.46 ± 0.05 | 59 ± 5 | 71 ± 2 | 19± 1 | 8 ± 2 | 1 ± 1 |
| PES-PIP-TMC | 11,500 ± 300 | 53 ± 1 | 0.58 ± 0.02 | 37 ± 5 | 74 ± 1 | 21 ± 2 | 1 ± 1 | 4 ± 1 |
| PES-PIP-TMC-PIP | 12,300 ± 400 | 52 ± 1 | 0.55 ± 0.02 | 30 ± 3 | 73 ± 1 | 21 ± 2 | 2 ± 1 | 4 ± 1 |
| Membrane | Permeance | Chemical Composition (XPS) [rel. atom-%] | ||||
|---|---|---|---|---|---|---|
| [L (m2 h bar)−1] | C | O | N | S | ||
| PES-PIP-TMC | before Soxhlet | 11,500 ± 300 | 74 ± 1 | 21 ± 2 | 1 ± 1 | 4 ± 1 |
| after Soxhlet | 13,100 ± 900 | 74 ± 3 | 21 ± 1 | 1 ± 1 | 5 ± 2 | |
| PES-PIP-TMC-PIP | before Soxhlet | 12,300 ± 400 | 73 ± 1 | 21 ± 2 | 2 ± 1 | 4 ± 1 |
| after Soxhlet | 12,600 ± 700 | 73 ± 2 | 20 ± 3 | 2 ± 1 | 6 ± 2 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breite, D.; Went, M.; Prager, A.; Kühnert, M.; Schulze, A. Reduction of Biofouling of a Microfiltration Membrane Using Amide Functionalities—Hydrophilization without Changes in Morphology. Polymers 2020, 12, 1379. https://doi.org/10.3390/polym12061379
Breite D, Went M, Prager A, Kühnert M, Schulze A. Reduction of Biofouling of a Microfiltration Membrane Using Amide Functionalities—Hydrophilization without Changes in Morphology. Polymers. 2020; 12(6):1379. https://doi.org/10.3390/polym12061379
Chicago/Turabian StyleBreite, Daniel, Marco Went, Andrea Prager, Mathias Kühnert, and Agnes Schulze. 2020. "Reduction of Biofouling of a Microfiltration Membrane Using Amide Functionalities—Hydrophilization without Changes in Morphology" Polymers 12, no. 6: 1379. https://doi.org/10.3390/polym12061379