Enhanced Emulsifying Ability of Deoxycholate through Dynamic Interaction with Layered Double Hydroxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of DCA−LDH
2.3. Preparation of the Lipid-Core Capsule Formulations

2.4. Characterization
3. Results and Discussion
3.1. Structural Analysis of DCA and DCA−LDH
3.2. Stability of DCA- and DCA−LDH-Containing Formulations in Acidic Condition
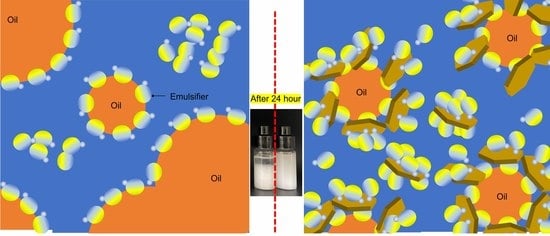
3.3. Colloidal Behavior of DCA- and DCA−LDH-Containing Formulations
3.4. Zeta Potential of DCA in Oil–in–Water Emulsions
3.5. Stability Monitoring by Time-Dependent Turbidity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Quinn, E.C.; Sickafus, K.E.; Ewing, R.C.; Baldinozzi, G.; Neuefeind, J.C.; Tucker, M.G.; Fuentes, A.F.; Drey, D.; Lang, M.K. Predicting short-range order and correlated phenomena in disordered crystalline materials. Sci. Adv. 2020, 6, eabc2758. [Google Scholar] [CrossRef]
- Boebinger, M.G.; Brea, C.; Ding, L.P.; Misra, S.; Olunloyo, O.; Yu, Y.; Xiao, K.; Lupini, A.R.; Ding, F.; Hu, G. The atomic drill bit: Precision controlled atomic fabrication of 2D materials. Adv. Mater. 2023, 2210116. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Gwak, G.-H.; Lee, M.; Paek, S.-M.; Oh, J.-M. Synthesis and structural analysis of ternary Ca–Al–Fe layered double hydroxides with different iron contents. Crystals 2021, 11, 1296. [Google Scholar] [CrossRef]
- Zhou, S.; Zeng, X.; Yan, X.; Xie, F.; Fahlman, B.D.; Wang, C.; Li, W. High aspect ratio copper nanowires and copper nanoparticles decorated by reduced graphene oxide for flexible transparent conductive electrodes. Appl. Surf. Sci. 2022, 604, 154597. [Google Scholar] [CrossRef]
- Chen, X.; Lv, H. Intelligent control of nanoparticle synthesis on microfluidic chips with machine learning. NPG Asia Mater. 2022, 14, 69. [Google Scholar] [CrossRef]
- Mansoor, S.; Shahid, S.; Ashiq, K.; Alwadai, N.; Javed, M.; Iqbal, S.; Fatima, U.; Zaman, S.; Sarwar, M.N.; Alshammari, F.H. Controlled growth of nanocomposite thin layer based on Zn-Doped MgO nanoparticles through Sol-Gel technique for biosensor applications. Inorg. Chem. Commun. 2022, 142, 109702. [Google Scholar] [CrossRef]
- Ditta, N.A.; Yaqub, M.; Nadeem, S.; Jamil, S.; Hassan, S.U.; Iqbal, S.; Javed, M.; Elkaeed, E.; Alzhrani, R.M.; Awwad, N.S. Electrochemical studies of LbL films with dawson type heteropolyanion glassy carbon electrode sensor modified for methyl parathion detection. Front. Mater. 2022, 9, 877683. [Google Scholar] [CrossRef]
- Salauddin, M.; Rana, S.S.; Rahman, M.T.; Sharifuzzaman, M.; Maharjan, P.; Bhatta, T.; Cho, H.; Lee, S.H.; Park, C.; Shrestha, K. Fabric-assisted MXene/silicone nanocomposite-based triboelectric nanogenerators for self-powered sensors and wearable electronics. Adv. Funct. Mater. 2022, 32, 2107143. [Google Scholar] [CrossRef]
- Al-Harbi, L.M.; Alsulami, Q.A.; Farea, M.; Rajeh, A. Tuning optical, dielectric, and electrical properties of Polyethylene oxide/Carboxymethyl cellulose doped with mixed metal oxide nanoparticles for flexible electronic devices. J. Mol. Struct. 2023, 1272, 134244. [Google Scholar] [CrossRef]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef]
- Davis, S.S.; Hadgraft, J.; Palin, K.J. Medical and pharmaceutical applications of emulsions. Encycl. Emuls. Technol. 1985, 2, 159–238. [Google Scholar]
- Parente, M.E.; Ares, G.; Manzoni, A.V. Application of two consumer profiling techniques to cosmetic emulsions. J. Sens. Stud. 2010, 25, 685–705. [Google Scholar] [CrossRef]
- Abdou, L.; El-Molla, M.; Hakeim, O.; El-Gammal, M.; Shamey, R. Synthesis of nanoscale binders through mini emulsion polymerization for textile pigment applications. Ind. Eng. Chem. Res. 2013, 52, 2195–2200. [Google Scholar] [CrossRef]
- Mulqueen, P. Recent advances in agrochemical formulation. Adv. Colloid Interface Sci. 2003, 106, 83–107. [Google Scholar] [CrossRef]
- Knowles, A. Recent developments of safer formulations of agrochemicals. Environmentalist 2008, 28, 35–44. [Google Scholar] [CrossRef]
- Liang, H.-N.; Tang, C.-H. pH-dependent emulsifying properties of pea [Pisum sativum (L.)] proteins. Food Hydrocoll. 2013, 33, 309–319. [Google Scholar] [CrossRef]
- Czaikoski, A.; Gomes, A.; Kaufmann, K.C.; Liszbinski, R.B.; de Jesus, M.B.; da Cunha, R.L. Lignin derivatives stabilizing oil-in-water emulsions: Technological aspects, interfacial rheology and cytotoxicity. Ind. Crops Prod. 2020, 154, 112762. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Sun, P.; Yang, C. Pickering emulsions stabilized by native starch granules. Colloids Surf. A 2013, 431, 142–149. [Google Scholar] [CrossRef]
- Gao, W.; Jiang, Z.; Du, X.; Zhang, F.; Liu, Y.; Bai, X.; Sun, G. Impact of surfactants on nanoemulsions based on fractionated coconut oil: Emulsification stability and In Vitro digestion. J. Oleo Sci. 2020, 69, 227–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [Green Version]
- Moradi, M.; Yamini, Y. Surfactant roles in modern sample preparation techniques: A review. J. Sep. Sci. 2012, 35, 2319–2340. [Google Scholar] [CrossRef]
- Mitra, S.; Dungan, S.R. Cholesterol solubilization in aqueous micellar solutions of quillaja saponin, bile salts, or nonionic surfactants. J. Agric. Food Chem. 2001, 49, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Garruti, G.; Baccetto, R.L.; Molina-Molina, E.; Bonfrate, L.; Portincasa, P.; Wang, D.Q. Bile acid physiology. Ann. Hepatol. 2018, 16, 4–14. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Maitra, U. Chemistry and biology of bile acids. Curr. Sci. 2004, 87, 1666–1683. [Google Scholar]
- Hylemon, P.B.; Zhou, H.; Pandak, W.M.; Ren, S.; Gil, G.; Dent, P. Bile acids as regulatory molecules. J. Lipid Res. 2009, 50, 1509–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keitel, V.; Kubitz, R.; Häussinger, D. Endocrine and paracrine role of bile acids. World J. Gastroenterol. 2008, 14, 5620. [Google Scholar] [CrossRef] [PubMed]
- Rzany, B.; Griffiths, T.; Walker, P.; Lippert, S.; McDiarmid, J.; Havlickova, B. Reduction of unwanted submental fat with ATX-101 (deoxycholic acid), an adipocytolytic injectable treatment: Results from a phase III, randomized, placebo-controlled study. Br. J. Dermatol. 2014, 170, 445–453. [Google Scholar] [CrossRef]
- Farina, G.A.; Cherubini, K.; de Figueiredo, M.A.Z.; Salum, F.G. Deoxycholic acid in the submental fat reduction: A review of properties, adverse effects, and complications. J. Cosmet. Dermatol. 2020, 19, 2497–2504. [Google Scholar] [CrossRef]
- Nie, B.; Park, H.M.; Kazantzis, M.; Lin, M.; Henkin, A.; Ng, S.; Song, S.; Chen, Y.; Tran, H.; Lai, R. Specific bile acids inhibit hepatic fatty acid uptake in mice. Hepatology 2012, 56, 1300–1310. [Google Scholar] [CrossRef] [Green Version]
- Madenci, D.; Egelhaaf, S. Self-assembly in aqueous bile salt solutions. Curr. Opin. Colloid Interface Sci. 2010, 15, 109–115. [Google Scholar] [CrossRef]
- Natalini, B.; Sardella, R.; Gioiello, A.; Ianni, F.; Di Michele, A.; Marinozzi, M. Determination of bile salt critical micellization concentration on the road to drug discovery. J. Pharm. Biomed. Anal. 2014, 87, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Moroi, Y. Micelle formation of sodium deoxycholate and sodium ursodeoxycholate (Part 1). Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2002, 1580, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Galantini, L.; di Gregorio, M.C.; Gubitosi, M.; Travaglini, L.; Tato, J.V.; Jover, A.; Meijide, F.; Tellini, V.H.S.; Pavel, N.V. Bile salts and derivatives: Rigid unconventional amphiphiles as dispersants, carriers and superstructure building blocks. Curr. Opin. Colloid Interface Sci. 2015, 20, 170–182. [Google Scholar] [CrossRef]
- Nesterenko, A.; Drelich, A.; Lu, H.; Clausse, D.; Pezron, I. Influence of a mixed particle/surfactant emulsifier system on water-in-oil emulsion stability. Colloids Surf. Physicochem. Eng. Asp. 2014, 457, 49–57. [Google Scholar] [CrossRef]
- Wu, J.; Ma, G.H. Recent Studies of Pickering Emulsions: Particles Make the Difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Choy, J.-H.; Choi, S.-J.; Oh, J.-M.; Park, T. Clay minerals and layered double hydroxides for novel biological applications. Appl. Clay Sci. 2007, 36, 122–132. [Google Scholar] [CrossRef]
- Suzuki, H.; Ogawa, M.; Hironaka, K.; Ito, K.; Sunada, H. A nifedipine coground mixture with sodium deoxycholate. II. Dissolution characteristics and stability. Drug Dev. Ind. Pharm. 2001, 27, 951–958. [Google Scholar] [CrossRef]
- Xie, J.; Yamaguchi, T.; Oh, J.-M. Synthesis of a mesoporous Mg–Al–mixed metal oxide with P123 template for effective removal of Congo red via aggregation-driven adsorption. J. Solid State Chem. 2021, 293, 121758. [Google Scholar] [CrossRef]
- Mokhtar, M.; Inayat, A.; Ofili, J.; Schwieger, W. Thermal decomposition, gas phase hydration and liquid phase reconstruction in the system Mg/Al hydrotalcite/mixed oxide: A comparative study. Appl. Clay Sci. 2010, 50, 176–181. [Google Scholar] [CrossRef]
- Kamel, A.H.; Ezzat, S.; Ahmed, M.A.; Amr, A.E.-G.E.; Almehizia, A.A.; Al-Omar, M.A. Modified potentiometric screen-printed electrodes based on imprinting character for sodium deoxycholate determination. Biomolecules 2020, 10, 251. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Wang, S.; Du, N.; Zhang, R.; Hou, W. Facile synthesis of deoxycholate intercalated layered double hydroxide nanohybrids via a coassembly process. J. Solid State Chem. 2013, 203, 181–186. [Google Scholar] [CrossRef]
- Sun, X.; Xin, X.; Tang, N.; Guo, L.; Wang, L.; Xu, G. Manipulation of the gel behavior of biological surfactant sodium deoxycholate by amino acids. J. Phys. Chem. B 2014, 118, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Luthra, S.; Krzyzaniak, J.F.; Agra-Kooijman, D.M.; Kumar, S.; Byrn, S.R.; Shalaev, E.Y. Crystalline, liquid crystalline, and isotropic phases of sodium deoxycholate in water. J. Pharm. Sci. 2011, 100, 4836–4844. [Google Scholar] [CrossRef] [PubMed]
- Carswell, A.D.; Lowe, A.M.; Wei, X.; Grady, B.P. CMC determination in the presence of surfactant-adsorbing inorganic particulates. Colloids Surf. A 2003, 212, 147–153. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, M.; Jiang, J.; Cui, Z.; Xia, W.; Binks, B.P. Conversion of bile salts from inferior emulsifier to efficient smart emulsifier assisted by negatively charged nanoparticles at low concentrations. Chem. Sci. 2021, 12, 11845–11850. [Google Scholar] [CrossRef]
- Zheng, B.; Zheng, B.; Carr, A.J.; Yu, X.; McClements, D.J.; Bhatia, S.R. Emulsions stabilized by inorganic nanoclays and surfactants: Stability, viscosity, and implications for applications. Inorg. Chim. Acta 2020, 508, 119566. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic-Curic, N.; Scheglmann, D.; Albrecht, V.; Fahr, A. Temoporfin-loaded invasomes: Development, characterization and In Vitro skin penetration studies. J. Control. Release 2008, 127, 59–69. [Google Scholar] [CrossRef]
- Badran, M. Formulation and In Vitro evaluation of flufenamic acid loaded deformable liposomes for improved skin delivery. Dig. J. Nanomater. Biostructures 2014, 9, 83–91. [Google Scholar]
- Huh, K.M.; Lee, K.Y.; Kwon, I.C.; Kim, Y.-H.; Kim, C.; Jeong, S.Y. Synthesis of triarmed poly (ethylene oxide)− deoxycholic acid conjugate and its micellar characteristics. Langmuir 2000, 16, 10566–10568. [Google Scholar] [CrossRef]
- Dayan, S.H.; Humphrey, S.; Jones, D.H.; Lizzul, P.F.; Gross, T.M.; Stauffer, K.; Beddingfield, F.C., III. Overview of ATX-101 (deoxycholic acid injection): A nonsurgical approach for reduction of submental fat. Dermatol. Surg. 2016, 42, S263–S270. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Tyner, K.M.; Dair, B.J.; Deschamps, J.R.; Medintz, I.L. Analyzing nanomaterial bioconjugates: A review of current and emerging purification and characterization techniques. Anal. Chem. 2011, 83, 4453–4488. [Google Scholar] [CrossRef] [PubMed]
- Meili, L.; Lins, P.; Zanta, C.; Soletti, J.; Ribeiro, L.; Dornelas, C.; Silva, T.; Vieira, M. MgAl-LDH/Biochar composites for methylene blue removal by adsorption. Appl. Clay Sci. 2019, 168, 11–20. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, Y.; Yang, J.; Zhang, L.; Hou, S.; Niu, X.; Zhu, J.; Huang, Y.; Sun, X.; Xu, Z.P. Efficient immunotherapy of drug-free layered double hydroxide nanoparticles via neutralizing excess acid and blocking tumor cell autophagy. ACS Nano 2022, 16, 12036–12048. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yin, P.; Yang, H. Superb adsorption of organic dyes from aqueous solution on hierarchically porous composites constructed by ZnAl-LDH/Al(OH)3 nanosheets. Microporous Mesoporous Mater. 2018, 259, 123–133. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Domingues, P.; Silva, T.; Almeida, A.; Zheludkevich, M.; Tedim, J.; Ferreira, M.; Cunha, A. Antimicrobial activity of 2-mercaptobenzothiazole released from environmentally friendly nanostructured layered double hydroxides. J. Appl. Microbiol. 2017, 122, 1207–1218. [Google Scholar] [CrossRef]
- Cadete, A.; Figueiredo, L.; Lopes, R.; Calado, C.; Almeida, A.; Gonçalves, L. Development and characterization of a new plasmid delivery system based on chitosan–sodium deoxycholate nanoparticles. Eur. J. Pharm. Sci. 2012, 45, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Xie, C.; Ping, Q. Preparation of stable solid lipid nanoparticles (SLNs) suspension with combined surfactants. J. China Pharm. Univ. 2005, 6, 417–422. [Google Scholar]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Kabal’Nov, A.; Pertzov, A.; Shchukin, E. Ostwald ripening in two-component disperse phase systems: Application to emulsion stability. Colloids Surf. 1987, 24, 19–32. [Google Scholar] [CrossRef]
- LeMarchand, C.; Couvreur, P.; Vauthier, C.; Costantini, D.; Gref, R. Study of emulsion stabilization by graft copolymers using the optical analyzer Turbiscan. Int. J. Pharm. 2003, 254, 77–82. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.F.; Lu, L.J.; Li, M.X.; Xu, J.C.; Deng, H.P. Turbiscan Lab (R) Expert analysis of the biological demulsification of a water-in-oil emulsion by two biodemulsifiers. J. Hazard. Mater. 2011, 190, 214–221. [Google Scholar] [CrossRef]
- Wilde, P.J. Improving emulsion stability through selection of emulsifiers and stabilizers. Ref. Modul. Food Sci. 2019. [Google Scholar] [CrossRef]
- Sarkar, A.; Ye, A.; Singh, H. On the role of bile salts in the digestion of emulsified lipids. Food Hydrocoll. 2016, 60, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Mbela, T.; Verschueren, E. The influence of additives on physical properties of emulsions prepared using lecithins and non-ionic surfactants. J. Pharm. Belg. 1997, 52, 110–116. [Google Scholar]
- Yunomiya, Y.; Kunitake, T.; Tanaka, T.; Sugihara, G.; Nakashima, T. Micellization of mixed system of bile salt and nonionic surfactant: Sodium deoxycholate and decanoyl-N-methylglucamide. J. Colloid Interface Sci. 1998, 208, 1–5. [Google Scholar] [CrossRef]
- Wang, J.; Yang, F.; Tan, J.; Liu, G.; Xu, J.; Sun, D. Pickering emulsions stabilized by a lipophilic surfactant and hydrophilic platelike particles. Langmuir 2010, 26, 5397–5404. [Google Scholar] [CrossRef]
- Roland, I.; Piel, G.; Delattre, L.; Evrard, B. Systematic characterization of oil-in-water emulsions for formulation design. Int. J. Pharm. 2003, 263, 85–94. [Google Scholar] [CrossRef]
- Kaombe, D.D.; Lenes, M.; Toven, K.; Glomm, W.R. Turbiscan as a Tool for Studying the Phase Separation Tendency of Pyrolysis Oil. Energy Fuels 2013, 27, 1446–1452. [Google Scholar] [CrossRef]
- De Paola, M.G.; Calabrò, V.; De Simone, M. Light Scattering Methods to Test Inorganic PCMs for Application in Buildings. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Riga, Latvia, 27–29 September 2017. [Google Scholar]







| Formulation | DCA | CCT | Co-Emulsifier | Water | Formulation | DCA−LDH | CCT | Co-Emulsifier | Water |
|---|---|---|---|---|---|---|---|---|---|
| F1 | 1% | 2% | - | 97% | F1′ | 2% | 2% | - | 96% |
| F2 | 1% | 2% | PS 60 2% | 95% | F2′ | 2% | 2% | PS 60 2% | 94% |
| F3 | 1% | 2% | HL 2% | 95% | F3′ | 2% | 2% | HL 2% | 94% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Lee, K.; Park, H.; Jung, H.; Oh, J.-M. Enhanced Emulsifying Ability of Deoxycholate through Dynamic Interaction with Layered Double Hydroxide. Nanomaterials 2023, 13, 567. https://doi.org/10.3390/nano13030567
Xie J, Lee K, Park H, Jung H, Oh J-M. Enhanced Emulsifying Ability of Deoxycholate through Dynamic Interaction with Layered Double Hydroxide. Nanomaterials. 2023; 13(3):567. https://doi.org/10.3390/nano13030567
Chicago/Turabian StyleXie, Jing, Kyounghyoun Lee, Hyeonjin Park, Hyun Jung, and Jae-Min Oh. 2023. "Enhanced Emulsifying Ability of Deoxycholate through Dynamic Interaction with Layered Double Hydroxide" Nanomaterials 13, no. 3: 567. https://doi.org/10.3390/nano13030567