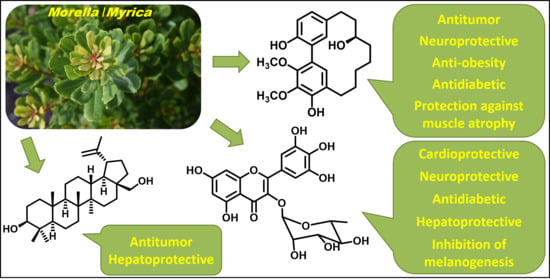
Phytochemicals with Added Value from Morella and Myrica Species
Abstract
:1. Introduction
2. Biological Activities Exhibited by Secondary Metabolites from Morella and Myrica Species
2.1. In Vitro Activities
2.2. In Vivo Tests
3. Other Phytochemicals Identified in Morella and Myrica Species
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-OHDA ACE-1 | 6-hydroxydopamine Angiotensin-converting-enzyme 1 |
| AGE | Advanced glycation end products |
| AICAR | 5-Aminoimidazole-4-carboxamide ribonucleotide |
| Akt | Protein kinase B |
| ALT | Alanine transaminase |
| AMPK aP2 | Adenosine monophosphate-activated protein kinase Adipocyte protein 2 |
| AST | Aspartate transaminase |
| ATP | Adenosine tri-phosphate |
| α-SMA | alpha smooth muscle actin |
| Bax | Bcl-2-associated X |
| Bcl-2 | B-cell lymphoma 2 |
| C/EBPα | CCAAT/enhancer-binding-protein-α |
| COX-2 | Cycloxygenase-2 |
| CYP2E1 | Cytochrome P450 2E1 |
| DNP | 2,4-dinitrophenol |
| EC50 | Half maximal effective concentration |
| ERK | Extracellular-signal-regulated kinase |
| FoxOs | Forkhead box O3 |
| γ-GCS | Gamma-glutamylcysteine synthetase |
| GSK-3β | Glycogen synthase kinase 3 beta |
| HDL | High-density lipoprotein |
| HFD | High-fat diet |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| HMGB1 | High mobility group box 1 protein |
| HO-1 | Heme oxygenase 1 |
| HUVECs | Human umbilical vein endothelial cells |
| IC50 | Half maximal inhibitory concentration |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| iNOS | Nitric oxide synthase |
| IRS-1 | Insulin receptor substrate 1 |
| IKK-β | I-kappa-B-kinase beta |
| JNK | c-Jun NH2-terminal cinase |
| LDH | Lactate Dehydrogenase |
| LDL | Low-density lipoprotein |
| LKB1 | Tumor suppressor serine/threonine-protein kinase |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MIC | Minimum inhibitory concentration |
| mTOR | mammalian target of rapamycin |
| MuRF1 | Muscle RING-finger protein-1 |
| MyD88 | Myeloid differentiation primary response 88 |
| NA | Neuraminidase |
| NF-KB | Nuclear factor kappa B |
| NQO-1 | NAD(P)H Quinone Dehydrogenase 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| Ox-LDL | Oxidized low-density lipoprotein |
| ROS | Reactive oxygen species |
| PGC-1α | Peroxisome proliferator-activated receptor-gamma coactivator |
| PI3-K | Phosphoinositide 3-kinases |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PSD-45 | Postsynaptic density protein-45 |
| SIRT1 | Sirtuin 1 |
| SREB-1 | Sterol regulatory element-binding transcription factor 1 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TGF-β1 | Transforming growth factor beta 1 |
| TH | Tyrosine hydroxylase |
| TG | Triglycerides |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor α |
| VEGF | Vascular endothelial growth factor |
References
- Seca, A.M.L.; Moujir, L.M. Natural compounds: A dynamic field of applications. Appl. Sci. 2020, 10, 4025. [Google Scholar] [CrossRef]
- Kroymann, J. Natural diversity and adaptation in plant secondary metabolism. Curr. Opin. Plant Biol. 2011, 14, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, D.; Cholarajan, A.; Raja, S.S.S.; Vijayakumar, R. An introductory chapter: Secondary metabolites. In Secondary Metabolites—Sources and Applications; Vijayakumar, R., Raja, S.S.S., Eds.; IntechOpen: London, UK, 2018; pp. 13–21. [Google Scholar] [CrossRef] [Green Version]
- Austen, N.; Walker, H.J.; Lake, J.A.; Phoenix, G.K.; Cameron, D.D. The regulation of plant secondary metabolism in response to abiotic stress: Interactions between heat shock and elevated CO2. Front. Plant Sci. 2019, 10, 1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019; WHO Press: Geneva, Switzerland, 2019; Available online: https://www.who.int/traditional-complementary-integrativemedicine/WhoGlobalReportOnTraditionalAndComplementaryMedicine2019.pdf?ua=1 (accessed on 1 September 2020).
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Gtari, M.; Dawson, J.O. An overview of actinorhizal plants in Africa. Funct. Plant Biol. 2011, 38, 653–661. [Google Scholar] [CrossRef]
- Yanthan, M.; Misra, A.K. Molecular approach to the classification of medicinally important actinorhizal genus Myrica. Indian J. Biotechnol. 2013, 12, 133–136. [Google Scholar]
- Huguet, V.; Gouy, M.; Normand, P.; Zimpfer, J.F.; Fernandez, M.P. Molecular phylogeny of Myricaceae: A reexamination of host-symbiont specificity. Mol. Phylogen. Evol. 2005, 34, 557–568. [Google Scholar] [CrossRef]
- Macdonald, A.D. The morphology and relationships of the Myricaceae. In Evolution, Systematics, and Fossil History of the Hamamelidae; Crane, P.R., Blackmore, S., Eds.; Oxford University Press: Oxford, UK, 1989; Volume 2, pp. 147–165. [Google Scholar]
- Staples, G.W.; Imada, C.T.; Herbst, D.R. New Hawaiian plant records for 2000. Bish. Mus. Occas. Pap. 2002, 68, 3–18. [Google Scholar]
- Herbert, J. Systematic and Biogeography of Myricaceae. Ph.D. Thesis, University of St Andrews, St Andrews, UK, 2005. [Google Scholar]
- Silva, B.J.C.; Seca, A.M.L.; Barreto, M.D.C.; Pinto, D.C.G.A. Recent breakthroughs in the antioxidant and anti-inflammatory effects of Morella and Myrica species. Int. J. Mol. Sci. 2015, 16, 17160–17180. [Google Scholar] [CrossRef] [Green Version]
- Nhiem, N.X.; van Kiem, P.; van Minh, C.; Tai, B.H.; Cuong, N.X.; Thu, V.K.; Anh, H.L.T.; Jo, S.-H.; Jang, H.-D.; Kwon, Y.-I.; et al. A new monoterpenoid glycoside from Myrica esculenta and the inhibition of angiotensin I-converting enzyme. Chem. Pharm. Bull. 2010, 58, 1408–1410. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.; Siddiqui, M.A.; Khan, M.M.; Ajmal, M.; Ahsan, R.; Rahaman, M.A.; Ahmad, M.A.; Arshad, M.; Khushtar, M. Current pharmacological trends on myricetin. Drug Res. 2020, 70, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Sil, P.C. Arjunolic acid: A new multifunctional therapeutic promise of alternative medicine. Biochimie 2013, 95, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhao, M.; Li, C.; Chang, Q.; Liu, X.; Liao, Y.; Pan, R. Study on the material basis of neuroprotection of Myrica rubra bark. Molecules 2019, 24, 2993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yamada, S.; Ogihara, E.; Kurita, M.; Banno, N.; Qu, W.; Feng, F.; Akihisa, T. Biological activities of triterpenoids and phenolic compounds from Myrica cerifera bark. Chem. Biodivers. 2016, 13, 1601–1609. [Google Scholar] [CrossRef]
- Ting, Y.-C.; Ko, H.-H.; Wang, H.-C.; Peng, C.-F.; Chang, H.-S.; Hsieh, P.-C.; Chen, I.-S. Biological evaluation of secondary metabolites from the roots of Myrica adenophora. Phytochemistry 2014, 103, 89–98. [Google Scholar] [CrossRef]
- Tene, M.; Tane, P.; Connolly, J.D. Triterpenoids and diarylheptanoids from Myrica arborea. Biochem. Syst. Ecol. 2008, 36, 872–874. [Google Scholar] [CrossRef]
- Yu, Y.-F.; Lu, Q.; Guo, L.; Mei, R.-Q.; Liang, H.-X.; Luo, D.-Q.; Cheng, Y.-X. Myricananone and myricananadiol: Two new cyclic ‘diarylheptanoids’ from the roots of Myrica nana. Helv. Chim. Acta 2007, 90, 1691–1696. [Google Scholar] [CrossRef]
- Nagai, M.; Sakurai, N.; Yumoto, N.; Nagumo, S.; Seo, S. Oleanane acid from Myrica cerifera. Chem. Pharm. Bull. 2000, 48, 1427–1428. [Google Scholar] [CrossRef] [Green Version]
- Joshi, B.S.; Pelletier, S.W.; Newton, M.G.; Lee, D.; McGaughey, G.B.; Puar, M.S. Extensive 1D, 2D NMR spectra of some [7.0]metacyclophanes and X-ray analysis of (2)-myricanol. J. Nat. Prod. 1996, 59, 759–764. [Google Scholar] [CrossRef]
- Nagai, M.; Dohi, J.; Morihara, M.; Sakurai, N. Diarylheptanoids from Myrica gale var. tomentosa and revised structure of porson. Chem. Pharm. Bull. 1995, 43, 1674–1677. [Google Scholar] [CrossRef] [Green Version]
- Dai, G.; Tong, Y.; Chen, X.; Ren, Z.; Yang, F. In vitro anticancer activity of myricanone in human lung adenocarcinoma A549 cells. Chemotherapy 2014, 60, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, H.; Fujita, Y.; Banno, N.; Watanabe, K.; Kimura, Y.; Manosroi, A.; Manosroi, J.; Akihisa, T. Three new cyclic diarylheptanoids and other phenolic compounds from the bark of Myrica rubra and their melanogenesis inhibitory and radical scavenging activities. J. Oleo Sci. 2010, 59, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, A.; Das, J.; Das, S.; Samadder, A.; Khuda-Bukhsh, A.R. Anticancer potential of myricanone, a major bioactive component of Myrica cerifera: Novel signaling cascade for accomplishing apoptosis. J. Acupunct. Meridian Stud. 2013, 6, 188–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Dong, S.; Wang, Y.; Lu, Q.; Zhong, H.; Du, G.; Zhang, L.; Cheng, Y. Cyclic diarylheptanoids from Myrica nana inhibiting nitric oxide release. Bioorg. Med. Chem. 2008, 16, 8510–8515. [Google Scholar] [CrossRef]
- Tene, M.; Wabo, H.K.; Kamnaing, P.; Tsopmo, A.; Tane, P.; Ayafor, J.F.; Sterner, O. Diarylheptanoids from Myrica arborea. Phytochemistry 2000, 54, 975–978. [Google Scholar] [CrossRef]
- Shen, S.; Liao, Q.; Liu, J.; Pan, R.; Lee, S.M.; Lin, L. Myricanol rescues dexamethasone-induced muscle dysfunction via a sirtuin 1-dependent mechanism. J. Cachexia Sarcopenia Muscle 2019, 10, 429–444. [Google Scholar] [CrossRef]
- Shen, S.; Liao, Q.; Feng, Y.; Liu, J.; Pan, R.; Lee, S.M.-Y.; Lin, L. Myricanol mitigates lipid accumulation in 3T3-L1 adipocytes and high fat diet-fed zebrafish via activating AMP-activated protein kinase. Food Chem. 2019, 270, 305–314. [Google Scholar] [CrossRef]
- Chen, P.; Lin, X.; Yang, C.-H.; Tang, X.; Chang, Y.-W.; Zheng, W.; Luo, L.; Xu, C.; Chen, Y.-H. Study on chemical profile and neuroprotective activity of Myrica rubra leaf extract. Molecules 2017, 22, 1226. [Google Scholar] [CrossRef] [Green Version]
- Dai, G.H.; Meng, G.M.; Tong, Y.L.; Chen, X.; Ren, Z.M.; Wang, K.; Yang, F. Growth-inhibiting and apoptosis-inducing activities of Myricanol from the bark of Myrica rubra in human lung adenocarcinoma A549 cells. Phytomedicine 2014, 21, 1490–1496. [Google Scholar] [CrossRef]
- Malterud, K.E.; Anthonsen, T.; Hjortas, J. 14-Oxa-[7.1]-metapara-cyclophanes from Myrica gale L., a new class of natural products. Tetrahedron Lett. 1976, 17, 3069–3072. [Google Scholar] [CrossRef]
- Morihara, M.; Sakurai, N.; Inoue, T.; Kawai, K.-I.; Nagai, M. Two novel diarylheptanoid glucosides from Myrica gale var. tomentosa and absolute structure of plane-chiral galeon. Chem. Pharm. Bull. 1997, 45, 820–823. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Cheng, B.; Liu, X.; Li, Y.; Hou, J.; Chen, S.; Chen, J.; Li, S. Screening of α-glucosidase inhibitors from Houttuynia cordata and evaluation of the binding mechanisms. ChemistrySelect 2020, 5, 8440–8446. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, L.; Cao, Y.; Li, J.; Atanasov, A.G.; Huang, L. Anti-neuraminidase activity of chemical constituents of Balanophora involucrata. Biomed. Chromatogr. 2020, 34, 4949. [Google Scholar] [CrossRef] [PubMed]
- Calassara, L.L.; Pinto, S.C.; Condack, C.P.M.; Leite, B.F.; Nery, L.C.D.E.S.; Tinoco, L.W.; Aguiar, F.A.; Leal, I.C.R.; Martins, S.M.; Silva, L.L.D.; et al. Isolation and characterization of flavonoids from Tapirira guianensis leaves with vasodilatory and myeloperoxidase-inhibitory activities. Nat. Prod. Res. 2020, 25, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Aldana, J.A.; De Grandis, R.A.; Nicolella, H.; Guissoni, A.P.P.; Squarisi, I.; Arruda, C.; Ribeiro, V.P.; Tavares, D.C.; Barcelos, G.R.M.; Antunes, L.M.G.; et al. Evaluation of cytoprotective effects of compounds isolated from Copaifera langsdorffii Desf. against induced cytotoxicity by exposure to methylmercury and lead. Nat. Prod. Res. 2020, 34, 2528–2532. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Lebar, M.D.; Jinwal, U.K.; Abisambra, J.F.; Koren, J.; Blair, L.; O’Leary, J.C.; Davey, Z.; Trotter, J.; Johnson, A.G.; et al. The diarylheptanoid (+)−aR,11S-myricanol and two flavones from bayberry (Myrica cerifera) destabilize the microtubule-associated protein Tau. J. Nat. Prod. 2011, 74, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.H.; Kim, D.H.; Kim, M.H.; Oh, M.H.; Kim, S.R.; Park, K.J.; Lee, M.W. Flavonoid constituents in the leaves of Myrica rubra Sieb. et Zucc. with anti-inflammatory activity. Arch. Pharmacal. Res. 2013, 36, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Rao, G.S.; Kapadia, G.J. Isolation of myricadiol, myricitrin, taraxerol, and taraxerone from Myrica cerifera L. root bark. J. Pharm. Sci. 1974, 63, 958–959. [Google Scholar] [CrossRef]
- Wang, M.; Sun, G.-B.; Du, Y.-Y.; Tian, Y.; Liao, P.; Liu, X.-S.; Ye, J.-X.; Sun, X.-B. Myricitrin protects cardiomyocytes from hypoxia/reoxygenation injury: Involvement of heat shock protein 90. Front. Pharmacol. 2017, 8, 353. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Shen, Q.; Chen, Y.; Pan, R.; Kuang, S.; Liu, G.; Sun, G.; Sun, X. Myricitrin alleviates oxidative stress-induced inflammation and apoptosis and protects mice against diabetic cardiomyopathy. Sci. Rep. 2017, 7, 44239. [Google Scholar] [CrossRef]
- Qin, M.; Luo, Y.; Meng, X.-B.; Wang, M.; Wang, H.-W.; Song, S.-Y.; Ye, J.-X.; Pan, R.-L.; Yao, F.; Wu, P.; et al. Myricitrin attenuates endothelial cell apoptosis to prevent atherosclerosis: An insight into PI3K/Akt activation and STAT3 signaling pathways. Vasc. Pharmacol. 2015, 70, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Feng, L.; Shen, Y.; Su, H.; Li, Y.; Zhuang, J.; Zhang, L.; Zheng, X. Myricitrin inhibits acrylamide-mediated cytotoxicity in human Caco-2 cells by preventing oxidative stress. BioMed Res. Int. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Xuan, Z.-H.; Tian, S.; He, G.-R.; Du, G.-H. Myricitrin attenuates 6-hydroxydopamine-induced mitochondrial damage and apoptosis in PC12 cells via inhibition of mitochondrial oxidation. J. Funct. Foods 2013, 5, 337–345. [Google Scholar] [CrossRef]
- Manaharan, T.; Appleton, D.; Cheng, H.M.; Palanisamy, U.D. Flavonoids isolated from Syzygium aqueum leaf extract as potential antihyperglycaemic agents. Food Chem. 2012, 132, 1802–1807. [Google Scholar] [CrossRef]
- Fang, J.; Paetz, C.; Schneider, B. C-methylated flavanones and dihydrochalcones from Myrica gale seeds. Biochem. Syst. Ecol. 2011, 39, 68–70. [Google Scholar] [CrossRef]
- Oracz, K.; Voegele, A.; Tarkowská, D.; Jacquemoud, D.; Turečková, V.; Urbanová, T.; Strnad, M.; Sliwinska, E.; Leubner-Metzger, G. Myrigalone A inhibits Lepidium sativum seed germination by interference with gibberellin metabolism and apoplastic superoxide production required for embryo extension growth and endosperm rupture. Plant. Cell Physiol. 2012, 53, 81–95. [Google Scholar] [CrossRef] [Green Version]
- Popovici, J.; Bertrand, C.; Jacquemoud, D.; Bellvert, F.; Fernandez, M.P.; Comte, G.; Piola, F. An allelochemical from Myrica gale with strong phytotoxic activity against highly invasive Fallopia x bohemica taxa. Molecules 2011, 16, 2323–2333. [Google Scholar] [CrossRef]
- Mathiesen, L.; Malterud, K.; Sund, R. Antioxidant activity of fruit exudate and C-methylated dihydrochalcones from Myrica gale. Planta Med. 1995, 61, 515–518. [Google Scholar] [CrossRef]
- Mathiesen, L.; Malterud, K.E.; Sund, R.B. Uncoupling of respiration and inhibition of ATP synthesis in mitochondria by C-methylated flavonoids from Myrica gale L. Eur. J. Pharm. Sci. 1996, 4, 373–379. [Google Scholar] [CrossRef]
- Popovici, J.; Comte, G.; Bagnarol, Ã.; Alloisio, N.; Fournier, P.; Bellvert, F.; Bertrand, C.; Fernandez, M.P. Differential effects of rare specific flavonoids on compatible and incompatible strains in the Myrica gale-Frankia actinorhizal symbiosis. Appl. Environ. Microbiol. 2010, 76, 2451–2460. [Google Scholar] [CrossRef] [Green Version]
- Gafner, S.; Wolfender, J.-L.; Mavi, S.; Hostettmann, K. Antifungal and antibacterial chalcones from Myrica serrata. Planta Med. 1996, 62, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Malterud, K.E. C-Methylated dihydrochalcones from Myrica gale fruit exudate. Acta Pharm. Nordica 1992, 4, 65–68. [Google Scholar]
- Lin, Y.; Chen, H.; Hsieh, C.; Huang, Y.; Chang, I. Betulin inhibits mTOR and induces autophagy to promote apoptosis in human osteosarcoma cell lines. Environ. Toxicol. 2020, 35, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Wold, C.W.; Gerwick, W.H.; Wangensteen, H.; Inngjerdingen, K.T. Bioactive triterpenoids and water-soluble melanin from Inonotus obliquus (Chaga) with immunomodulatory activity. J. Funct. Foods 2020, 71, 104025. [Google Scholar] [CrossRef]
- Wan, Y.; Jiang, S.; Lian, L.-H.; Bai, T.; Cui, P.-H.; Sun, X.-T.; Jin, X.-J.; Wu, Y.-L.; Nan, J.-X. Betulinic acid and betulin ameliorate acute ethanol-induced fatty liver via TLR4 and STAT3 in vivo and in vitro. Int. Immunopharmacol. 2013, 17, 184–190. [Google Scholar] [CrossRef]
- Sakurawi, K.; Yasuda, F.; Tozyo, T.; Nakamura, M.; Sato, T.; Kikuchi, J.; Terui, Y.; Ikenishi, Y.; Iwata, T.; Takahashi, K.; et al. Endothelin receptor antagonist triterpenoid, myriceric acid A, isolated from Myrica cerifera, and structure activity relationships of its derivatives. Chem. Pharm. Bull. 1996, 44, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, M.; Mihara, S.-I.; Nakajima, S.; Ueda, M.; Nakamura, M.; Sakurai, K.-S. A novel non-peptide endothelin antagonist isolated from bayberry, Myrica cerifera. FEBS Lett. 1992, 305, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Koike, R.; Yamamoto, A.; Ukiya, M.; Fukatsu, M.; Banno, N.; Miura, M.; Motohashi, S.; Tokuda, H.; Akihisa, T. Glycosidic inhibitors of melanogenesis from leaves of Passiflora edulis. Chem. Biodivers. 2013, 10, 1851–1865. [Google Scholar] [CrossRef]
- Ganapathy, G.; Preethi, R.; Moses, J.A.; Anandharamakrishnan, C. Diarylheptanoids as nutraceutical: A review. Biocatal. Agric. Biotechnol. 2019, 19, 101109. [Google Scholar] [CrossRef]
- Gurd, B.J. Deacetylation of PGC-1α by SIRT1: Importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl. Physiol. Nutr. Metab. 2011, 36, 589–597. [Google Scholar] [CrossRef]
- Lee, D.; Goldberg, A.L. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J. Biol. Chem. 2013, 288, 30515–30526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gullett, N.P.; Hebbar, G.; Ziegler, T.R. Update on clinical trials of growth factors and anabolic steroids in cachexia and wasting. Am. J. Clin. Nutr. 2010, 91, 1143–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Nersesyan, A.; Kundi, M.; Waldherr, M.; Setayesh, T.; Mišík, M.; Wultsch, G.; Filipic, M.; Mazzaron Barcelos, G.R.; Knasmueller, S. Results of micronucleus assays with individuals who are occupationally and environmentally exposed to mercury, lead and cadmium. Mutat. Res. Rev. Mutat. Res. 2016, 770, 119–139. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Wohlbold, T.J.; Zheng, N.-Y.; Huang, M.; Huang, Y.; Neu, K.E.; Lee, J.; Wan, H.; Rojas, K.T.; Kirkpatrick, E.; et al. Influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. Cell 2018, 173, 417–429. [Google Scholar] [CrossRef] [Green Version]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial uncoupling: A key controller of biological processes in physiology and diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Liao, Q.; Zhang, T.; Pan, R.; Lin, L. Myricanol modulates skeletal muscle–adipose tissue crosstalk to alleviate high-fat diet-induced obesity and insulin resistance. Br. J. Pharmacol. 2019, 176, 3983–4001. [Google Scholar] [CrossRef] [Green Version]
- Dai, G.; Tong, Y.; Chen, X.; Ren, Z.; Ying, X.; Yang, F.; Chai, K. Myricanol induces apoptotic cell death and anti-tumor activity in non-small cell lung carcinoma in vivo. Int. J. Mol. Sci. 2015, 16, 2717–2731. [Google Scholar] [CrossRef]
- Domitrović, R.; Rashed, K.; Cvijanović, O.; Vladimir-Knežević, S.; Škoda, M.; Višnić, A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem. Biol. Interact. 2015, 230, 21–29. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Liu, M.; Cheng, X.; Li, W.-H.; Zhang, S.-S.; Wang, Y.-H.; Du, G.-H. Myricitrin blocks activation of NF-κB and MAPK signaling pathways to protect nigrostriatum neuron in LPS-stimulated mice. J. Neuroimmunol. 2019, 337, 577049. [Google Scholar] [CrossRef] [Green Version]
- Bai, T.; Yang, Y.; Yao, Y.-L.; Sun, P.; Lian, L.-H.; Wu, Y.-L.; Nan, J.-X. Betulin alleviated ethanol-induced alcoholic liver injury via SIRT1/AMPK signaling pathway. Pharmacol. Res. 2016, 105, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Liu, J.; Zhang, J.; Zhu, D.; Wang, H.; Xiong, L.; Lee, Y.; Lian, K.; Xu, C.; Zhang, L.; et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int. J. Obes. 2016, 40, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourie, C.; Kim, E.; Waldvogel, H.; Wong, J.M.; Mcgregor, A.; Faull, R.L.M.; Montgomery, J.M. Differential changes in postsynaptic density proteins in postmortem huntington’s disease and parkinson’s disease human brains. J. Neurodegener. Dis. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Simmen, F.A.; Ronis, M.J.J.; Badger, T.M. Post-transcriptional regulation of sterol regulatory element-binding protein-1 by ethanol induces class I alcohol dehydrogenase in rat liver. J. Biol. Chem. 2004, 279, 28113–28121. [Google Scholar] [CrossRef] [Green Version]
- Santos, S.C.; Waterman, P.G. Condensed tannins from Myrica gale. Fitoterapia 2000, 71, 610–612. [Google Scholar] [CrossRef]
- Wang, J.-F.; Zhang, C.-L.; Lu, Q.; Yu, Y.-F.; Zhong, H.-M.; Long, C.-L.; Cheng, Y.-X. Three new diarylheptanoids from Myrica nana. Helv. Chim. Acta 2009, 92, 1594–1599. [Google Scholar] [CrossRef]
- Sakurai, N.; Hosono, Y.; Morihara, M.; Ishidi, J.; Kawai, K.-I. A new triterpenoid myricalactone and others from Myrica gale var. tomentosa. Yakugaku Zasshi 1997, 117, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, K.P.; Roy, A.C.; Dhar, M.L. Triterpenes from the bark of Myrica esculenta Buch.-Ham. Indian J. Chem. 1963, 1, 28–30. [Google Scholar]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A comprehensive review on the chemical constituents and functional uses of walnut (Juglans spp.) Husk. IJMS 2019, 20, 3920. [Google Scholar] [CrossRef] [Green Version]
- Seo, D.Y.; Lee, S.R.; Heo, J.-W.; No, M.-H.; Rhee, B.D.; Ko, K.S.; Kwak, H.-B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Q.; Yang, W.; Jia, Y.; Chen, X.; Gao, Q.; Bi, K. LC-MS determination and pharmacokinetic studies of ursolic acid in rat plasma after administration of the traditional chinese medicinal preparation Lu-Ying extract. Yakugaku Zasshi 2005, 125, 509–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Luo, S.; Zhang, Y.; Chen, Z. Development of a liquid chromatography–mass spectrometry method for the determination of ursolic acid in rat plasma and tissue: Application to the pharmacokinetic and tissue distribution study. Anal. Bioanal. Chem. 2011, 399, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
| Compound | Origin | Biological Activities |
|---|---|---|
Myricanone (1) | Methanol extract of Myrica rubra (Lour.) Siebold & Zucc. bark [17] Hexane extract of Morella cerifera (L.) Small (Myrica cerífera) bark [18] Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] Chloroform and methanol extract of Morella arborea (Hutch.) Cheek twigs [20] 95% EtOH extract of Morella nana (A. Chev.) J. Herb. roots [21] Hexane extract of Morella cerifera (L.) Small (Myrica cerífera) twigs [22] Ethanol 95% extract of Morella cerifera (L.) Small (Myrica cerífera) bark [23] Methanol extract of Myrica gale L. (Myrica gale var tormentosa L.) branches [24] | Anti-tuberculosis [19] Minimum inhibitory concentration (MIC) = >150 µg/mL (Ethambutol MIC = 6.25 µg/mL). Cytotoxic activity [25] A549 cell line EC50 = 3.22 µg/mL (Fluorouracil EC50 = not shown). Increase in apoptotic rate to 34.9% at 5.0 µg/mL (4.13% on untreated cells). Antimelanogenesis activity [26] Melanin content in B16 mouse melanoma cells at 25 µg/mL: 23.5 ± 3.4% (arbutin 25 µg/mL: 77.4 ± 2.9%). Cell viability at 25 µg/mL: 17.7 ± 1.7% (arbutin 25 µg/mL: 102.0 ± 1.5%). Cytotoxic activity [27] HepG2 cell line: EC50 = 32.46 µg/mL. WRL-68 cell line: at 100 µg/mL cell viability was 89.27% (non-tumor cell line). |
5-Deoxymyricanone (2) | Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] | Anti-tuberculosis [19] MIC = 25.8 µg/mL (Ethambutol MIC = 6.25 µg/mL). |
12-Hydroxymyricanone (3) | Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] Ethanol 80% extracts of Morella nana (A. Chev.) J. Herb. roots [28] Methanol extract of Myrica gale L. (Myrica gale var tormentosa L.) branches [24] | Anti-tuberculosis [19] MIC = 35.8 µg/mL (Ethambutol MIC = 6.25 µg/mL). |
Myricanol (4) | Methanol extract of Myrica rubra (Lour.) Siebold and Zucc. bark [17] Hexane extract of Morella cerifera (L.) Small (Myrica cerífera) bark [18] Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] Methanol extract of Myrica esculenta Buch.-Ham. Ex D. Don leaves [14] Chloroform and methanol extract of Morella arborea (Hutch.) Cheek twigs [20] 95% EtOH extract of Morella nana (A. Chev.) J. Herb. roots [21] Dichloromethane: methanol (1:1) extract of Morella arborea (Hutch.) Cheek bark and stem [29] Ethanol 95% extract of Morella cerifera (L.) Small bark [23] | Inhibition of muscle atrophy and dysfunction [30] C2C12 myotubes treated with 10 µM myricanol and dexamethasone showed: ↑ myosin heavy chain expression (0.89 against 0.33 on cells treated only with dexamethasone), ↓ atrogin-1 expression (1.53 against 2.31 on cells treated only with dexamethasone), ↓ MuRF1 expression (0.99 against 1.55 on cells treated only with dexamethasone), ↑ ATP production (5.84 nM/mg protein against 3.83 nM/mg protein on cells treated only with dexamethasone), ↑ mitochondrial content (116.38% against 68.12% on cells treated only with dexamethasone), ↑ mitochondrial O2 consumption (223.77 pmol/min against 166.59 pmol/min on cells treated only with dexamethasone). Mitigation of lipid accumulation in 3TS-L1 adipocytes [31] Treatment with 5 µM myricanol increased AMPK activation in 50% when compared to the untreated cells. Decrease of 28% in lipid accumulation when compared to the untreated cells. Neuroprotective effects [32] 80% increase in N2a cells viability treated with 0.84 mM and 100 mM H2O2 (against cells treated only with H2O2). 40% reduction of intracellular ROS in N2a cells treated with 0.84 mM and 100 mM H2O2 when compared with the cells treated only with H2O2. Intracellular calcium concentration of 777.81 nM in N2a cells treated with 0.84 mM and 100 mM H2O2 (3045.51 nM in cells treated only with H2O2). Antitumor activity [18] HL60 cell-line: IC50 = 5.3 ± 0.7 µM (Cisplatin EC50 = 4.2 ± 1.1 µM). A549 cell-line: IC50 = 16.5 ± 0.8 µM (Cisplatin EC50 = 18.4 ± 1.9 µM). SK-BR-3 cell-line: EC50 = 14.8 ± 5.5 µM (Cisplatin EC50 = 18.8 ± 0.6 µM). Anti tuberculosis [19] MIC = 30 µg/mL (Ethambutol MIC = 6.25 µg/mL). Cytotoxic activity [33] A549 cell line EC50 = 4.85 µg/mL (Fluorouracil EC50 = not shown). Antimelanogenesis activity [26] Melanin content in B16 mouse melanoma cells at 25 µg/mL: 3.8 ± 0.4% (arbutin 25 µg/mL: 77.4 ± 2.9%). Cell viability at 25 µg/mL: 8.8 ± 0.2% (arbutin 25 µg/mL: 102.0 ± 1.5%). |
Myricanol 11-sulphate (5) | Methanol extract of Myrica rubra (Lour.) Siebold and Zucc. bark [17] | Protection against glutamate-induced damage [17] PC12 cell line: 5 µM of myricanol 11-sulphate maintained 72.09 ± 2.09% of cell viability after 24 h exposure to glutamate. (Cells treated only with glutamate showed viability of 50%). |
Porson (6) | Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] 95% EtOH extract of Morella nana (A. Chev.) J. Herb. roots [21] Methanol extract of Myrica gale L. (Myrica gale var tormentosa L.) branches [24] Ethyl acetate extract of Myrica gale L. stems [34] | Anti-tuberculosis [19] MIC = 40 µg/mL (Ethambutol MIC = 6.25 µg/mL). |
Myricananin C (7) | Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] Ethanol 80% extract of Morella nana (A. Chev.) J. Herb. roots [28] | Anti-tuberculosis [19] MIC = 55.5 µg/mL (Ethambutol MIC = 6.25 µg/mL). |
(+)-Galeon (8) | Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] Methanol extract of Myrica gale L. (Myrica gale var tormentosa L.) branches [35] Ethyl acetate extract of Myrica gale L. stems [34] | Anti-tuberculosis [19] MIC = 15.0 µg/mL (Ethambutol MIC = 6.25 µg/mL). |
Quercitrin (9) | Methanol extract of Myrica rubra (Lour.) Siebold and Zucc. bark [17] Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] | α-Glucosidase Inhibition [36] IC50 = 0.231 ± 0.033 mg/mL (Acarbose IC50 = 1.457 ± 0.144 mg/mL). Antiviral Activity [37] Neuraminidase inhibition: IC50 = 311.76 μM (Oseltamivir acid IC50 = 280 μM). Myeloperoxidase inhibition [38] IC50 = 2.0 ± 0.2 μM Prevention of metal toxicity effects [39] Almost 100% HepG2 cell line viability when treated with 1 μM + 15 μM MeHg (50% viability when only treated with 15 μM MeHg). Almost 100% HepG2 cell line viability when treated with 1 μM + 40 μM Pb(NO3)2 (50% viability when only treated with 40 μM Pb(NO3)2). About 50% PC12 cell line viability when treated with 1 μM + 10 μM MeHg (65% viability when only treated with 10 μM MeHg). Almost 90% PC12 cell line viability when treated with 1 μM + 350 μM Pb(NO3)2 (60% viability when only treated with 350 μM Pb(NO3)2). Anti-tuberculosis [19] MIC = >150 µg/mL (Ethambutol MIC = 6.25 µg/mL). |
Myricitrin (10) | Methanol extract of Myrica rubra (Lour.) Siebold and Zucc. bark [17] Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] Toluene extract of the root-bark Morella cerifera (L.) Small (Myrica cerifera) [40] Methanol extract of Myrica esculenta Buch.-Ham. Ex D. Don leaves [14] 80% Acetone extract of Myrica rubra (Lour.) Siebold and Zucc. leaves [41] Benzene extract (after petroleum ether) of Morella cerifera (L.) Small (Myrica cerifera) root bark [42] | Protection against glutamate-induced damage [17] PC12 cell line: 10 µM of myricitrin maintained 73.15 ± 3.23% of cell viability after 24 h exposure to glutamate. (Cells treated only with glutamate showed only viability of 50%). Protection against hypoxia/reoxygenation (R/H) injury in cardiomyocyte [43] H9c2 cells pretreated for 12 h with 40 µM had a survival rate of 87.48 ± 7.68% (survival rate of untreated cells = 65.48 ± 6.42%). 50% decrease of LDH levels secreted to culture medium in the cells pretreated for 12 h with 40 µM when compared with untreated cells. Pretreatment with 40 µM inhibited intracellular ROS generation in 50% when compared to untreated cells. Reduction of 50% in the apoptotic rate on cells treated with 40 µM when compared with the untreated cells. Pretreatment with 40 µM decreased the caspase-3 activity in 25% when compared with the untreated cells. Pretreatment with 40 µM increased the Bcl-2/Bax expression ratio to 1.28 ± 0.31 (0.46 ± 0.12 in untreated cells. Protection against advanced glycation end products (AGEs) injury in cardiomyocyte [44] Pretreatment with 25 µg/mL led to H9c2 cells viability of 78.94 ± 4.52% (60.34 ± 6.52% when treated only with 400 μg/mL AGEs). Pretreatment with 25 µg/mL µM decreased phospho-IKK-β expression in about 40%, when compared with the untreated cells. Pretreatment with 25 µg/mL decreased TNF-α expression in about 50%, when compared with the untreated cells. Pretreatment with 25 µg/mL decreased intracellular ROS generation in about 50%, when compared with the untreated cells. Pretreatment with 25 µg/mL markedly attenuated the inhibition of NQO-1, γ -GCS, and HO-1 expression induced by AGEs. Pretreatment with 25 µg/mL decreased caspase-3 and caspase-9 activity in 3 and 2-fold, respectively, when compared with the untreated cells. Pretreatment with 25 µg/mL decreased apoptotic rate in about 7%, when compared with the untreated cells. Pretreatment with 25 µg/mL led to a 2-fold decrease in the expression of Bax compared with the untreated cells. Pretreatment with 25 µg/mL led to a 2-fold increase in the expression of Bcl-2 compared with the untreated cells. Pretreatment with 25 µg/mL led to a 33% decrease in the expression of collagen-1 compared with the untreated cells. Prevention of atherosclerosis [45] Pretreatment with 40 µM in oxidized low-density lipoprotein (ox-LDL) exposed human umbilical vein endothelial cells (HUVECs): Increased cell viability (70.75 ± 8.44% against 50.25 ± 7.95% in the untreated cells); Reduction of apoptotic cells (15.58 ± 4.65% against 23.89 ± 3.65% in the untreated cells); 3-fold reduction of intracellular ROS levels when compared with untreated cells; ~33% reduction on caspase-3 activity when compared with untreated cells. Anti-tuberculosis [19] MIC = >150 µg/mL (Ethambutol MIC = 6.25 µg/mL). Protection against acrylamide induced oxidative stress [46] Acrylamide induced Caco-2 cells treated with 40 µg/mL presented a viability of 80% (untreated cells had a viability of 50%). Neuroprotective activity [47] Pretreatment with 10µM prevents PC12 cell death induced by 6-hydroxydopamine (6-OHDA) (untreated cells presented 25% of cell mortality). Pretreatment with 10µM decreased cytochrome C release by almost 60% when compared with the untreated group. Pretreatment with 10µM decreased caspase-3 activity release by almost 50% when compared with the untreated group. Pretreatment with 10µM decreased the apoptotic rate activity by almost 50% when compared with the untreated group. Antidiabetic activity [48] α-glucosidase inhibition: IC50 = 1.1 ± 0.06 µM (acarbose: IC50 = 43 ± 1.6 µM). β-amylase inhibition: IC50 = 1.9 ± 0.02 µM. (acarbose: IC50 = 19 ± 1.6 µM). Antimelanogenesis activity [26] Melanin content in B16 mouse melanoma cells at 25 µg/mL: 69.9 ± 2.3% (arbutin 25 µg/mL: 77.4 ± 2.9%). Cell viability at 25 µg/mL: 102.9 ± 3.3% (arbutin 25 µg/mL: 102.0 ± 1.5%). |
Myrigalone A (11) | 50% acetone extract of Myrica gale L. seeds [49] Methanol extract of Myrica gale L. leaves and fruits [34], fruits [50], seeds [51] Diethyl ether extract of the fruit exudate of Myrica gale L. [52] | Uncoupling activity [53] Increases mitochondrial respiration rate by 87 ± 8 natoms O/min/mg (DNP 36 ± 3 natoms O/min/mg) at 45 µM. |
Myrigalone B (12) | 50% Acetone extract of Myrica gale L. seeds [49] Methanol extract of Myrica gale L. seeds [54] Dichloromethane extract of Morella serrata (Lam.) Killick leaves [55] Diethyl ether extract of the fruit exudate of Myrica gale L. [52] | Antidiabetic activity [48] α-glucosidase inhibition: IC50 = 19 ± 1.0 µM (acarbose: IC50 = 43 ± 1.6 µM). β-amylase inhibition: IC50 = 8.3 ± 1.3 µM (acarbose: IC50 = 19 ± 1.6 µM). Uncoupling activity [53] Increases mitochondrial respiration rate by 40 ± 10 natoms O/min/mg (DNP 36 ± 3 natoms O/min/mg) at 45µM. |
Myrigalone D (13) | 50% acetone extract of Myrica gale L. seeds [49] Methanol extract of Myrica gale L. seeds [54] and leaves [34] From the Myrica gale L. fruits [53] | Uncoupling activity [53] Increases mitochondrial respiration rate by 14 ± 2 natoms O/min/mg (DNP 36 ± 3 natoms O/min/mg) at 45 µM. |
Myrigalone H (14) | Diethyl ether extract of the fruit exudate of Myrica gale L. [52] Myrica gale L. fruit exudate [56] | Uncoupling activity [53] Increases mitochondrial respiration rate by 17 ± 3 natoms O/min/mg (DNP 36 ± 3 natoms O/min/mg) at 45 µM. |
Myrigalone G (15) | 50% acetone extract of Myrica gale L. seeds [49] Diethyl ether extract of the fruit exudate of Myrica gale L. [52] | Antidiabetic activity [48] α-glucosidase inhibition: IC50 = 7 ± 1.4 µM (acarbose: IC50 = 43 ± 1.6 µM). β-amylase inhibition: IC50 = 33 ± 6.6 µM (acarbose: IC50 = 19 ± 1.6 µM). Uncoupling activity [53] Increases mitochondrial respiration rate by 71 ± 5 natoms O/min/mg (DNP 36 ± 3 natoms O/min/mg) at 45 µM. |
Betulin (16) | Hexane extract of Morella cerifera (L.) Small (Myrica cerifera) bark [18] | Anti-osteosarcoma activity [57] MG-63 cell line: EC50 = 14.54 µM. HOS cell line: EC50 = 11.70 µM. Antitumor activity [58] NCI-H460 cell line: EC50 = 2.8 ± 0.4 µM (Podophyllotoxin: EC50 = 22 ± 6 µM). HT29-MTX cell line: EC50 = 1.6 ± 0.4 µM (Podophyllotoxin: EC50 = 24 ± 3 µM). Antitumor activity [18] HL60 cell-line: EC50 = 15.2 ± 2.3 µM (Cisplatin EC50 = 4.2 ± 1.1 µM). A549 cell-line: EC50 = 5.2 ± 3.0 µM (Cisplatin EC50 = 18.4 ± 1.9 µM). SK-BR-3 cell-line: EC50 = 3.1 ± 0.6 µM (Cisplatin EC50 = 18.8 ± 0.6 µM). Hepatoprotective activity [59] Pretreatment with 25 µM in ethanol-induced HSC-T6 cells: Decrease in the expression levels of collagen-I (35%), α-SMA (25%) and TLR4 (60%), when compared with untreated cells. ~30% increase in phosphorylation of STAT3, when compared with the untreated group. |
Myriceric acid A (17) | Methanol extract of Morella cerifera (L.) Small (Myrica cerifera) twigs [60] and branches [61] | Anti-hypertension activity [60] Endothelin 1 receptor antagonist: IC50 = 11 ± 2 nM. |
3β-trans-p-Coumaroyloxy-2α,23-dihydroxyolean-12-en-28-oic acid (18) | Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] | Anti-tuberculosis [19] MIC = 45 µg/mL (Ethambutol MIC = 6.25 µg/mL). |
(R)-4-(5-Hydroxy-7-(4-hydroxyphenyl)heptyl)-2-methoxyphenol (19) | Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] | Anti-tuberculosis [19] MIC = 52 µg/mL (Ethambutol MIC = 6.25 µg/mL). |
Corchoionoside C (20) | Methanol extract of Myrica esculenta Buch.-Ham. Ex D. Don leaves [14] | Angiotensin I-converting enzyme [14] 29.97 ± 4.77% ACE 1 inhibition at 100 µM (Captopril 88.64 ± 2.57 at 4.6 µM). |
(6S,9R)-Roseoside (21) | Methanol extract of Myrica esculenta Buch.-Ham. Ex D. Don leaves [14] | Antimelanogenesis activity [62] Inhibition of melanogenesis in B16 mouse melanoma cells at 100 µM: 62.7 ± 3.1% (arbutin 100 µM: 70.3 ± 5.5%). Cell viability at 100 µM: 95.0 ± 2.2% (arbutin 100 µM: 87.5 ± 2.8%). Angiotensin I-converting enzyme [14] 25.63 ± 1.35% ACE 1 inhibition at 100 µM (Captopril 88.64 ± 2.57 at 4.6 µM). |
Myricadenin A (22) | Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] | Anti-tuberculosis [19] MIC = 80.0 µg/mL (Ethambutol MIC = 6.25 µg/mL). |
| Compound | Model | Dose | Activity |
|---|---|---|---|
| Myricanol (4) | C57BL/6 mice | 5 mg/kg | Protection against muscle atrophy [30] Reduction of quadriceps muscle mass loss (1.36 ± 0.02% b/w against 1.18 ± 0.06% b/w on the untreated group). Reduction of gastrocnemius muscle mass loss (0.87 ± 0.08% b/w against 0.78 ± 0.05% b/w on the untreated group). Improvement of grip strength (120.58 ± 7.93 g against 70.90 ± 04.59 g on the untreated group). Increase in forced swim time (83.75 ± 15.19 s against 48.80 ± 11.43 s on the untreated group). Inhibition of muscle atrophy (~25% increase in muscle fiber diameter when compared with untreated group). |
| C57BL/6J | 25 mg/kg | Anti-obesity and diabetic activity [71] Reduction of body weight and body fat gain under high fat diet when compared with untreated group. Decrease in serum total cholesterol, triglycerides, LDL-cholesterol, and LDL/HDL ratio (~33%). 50% reduction of fasting insulin levels when compared with untreated group. ~2-fold increase in insulin sensitivity when compared with untreated group. Increased levels of phosphorylation of IRS-1 (2.5-fold), AKT (3-fold), and GSK-3β (1.5-fold) when compared with untreated group. 35% decrease in adipocyte diameter when compared with untreated group. ~33% increase in irisin serum levels when compared with untreated group. | |
| Zebrafish | 1 µM | Anti-obesity activity [31] 66% decrease in lipid accumulation under high-fat diet, when compared with the untreated group (positive control AICAR (5 µM): 75% decrease). ~70–80% reduction of PPAR-γ, C/EBPα, SREB-1 and aP2 expression when compared with the untreated group (similar values obtained with the positive control). | |
| BALB/c nude mice | 40 mg/kg | Antitumor activity [72] 39.4% reduction on A549 xenograft tumor volume after 14 days, when compared with untreated group. ~33% increase in the expression levels of Bax when compared with untreated group. Decrease in the expression levels of Bcl-2 (25%), vascular endothelial growth factor (VEGF) (20%), and Survivin (33%) when compared with untreated group. 20% increase on the number of apoptotic tumor cells when compared with untreated group. | |
| Myricitrin (10) | ApoE -/- mice | 50 mg/kg | Prevention of atherosclerosis [45] ~25% reduction of serum levels of Ox-LDL (similar reduction in the positive control group treated with 2 g/kg of probucol). 22% reduction on aortic wall thickness when compared to the untreated group. Complete inhibition of calcification on aortic arch. (Calcification observed in the untreated group). 26% reduction on atherosclerotic plaque area when compared with the untreated group. ~20% reduction of caspase-3 expression in aortic arch. |
| BALB/cN mice | 100 mg/kg | Hepatoprotective activity [73] Protection against CCl4 intoxication. Reduction in body weight change (−12.9 ± 0.8% against 19.6 ± 2.3% on the untreated group. Reduction of ALT serum levels (481 ± 53 U/L against 2126 ± 268 U/L on the untreated group). Reduction of AST serum levels (592 ± 74 U/L against 2538 ± 322 U/L on the untreated group). Reduction of liver lipids peroxidation. Increase on glutathione levels (46.1 ± 3.4 µmol/g protein against 28.6 ± 2.8 µmol/g protein on the untreated group). ~25% decrease in necrotic areas on the liver when compared with untreated group. 5-fold increase on CYP2E1 expression when compared with the untreated group. | |
| BALB/c mice | 300 mg/kg | Protection against diabetic cardiomyopathy [44] ~20–25% improvement in cardiac function of diabetic mice when compared with untreated group. Decrease on abnormalities in the arrangement of cardiac fibers and morphology of cardiomyocytes when compared with untreated group. Inhibition of collagen network destruction and reduction of fibrotic alterations of the hearth. 2-fold reduction in the expression of TGF-β1 when compared with the untreated group. 8-fold reduction in the expression levels of collagen-1. Reduction on serum levels of IL-6 (35.72 pg/mL against 112.41 pg/mL in the untreated group). Reduction on serum levels of TNF-α (24.83 pg/mL against 56.21 pg/mL in the untreated group). Decrease of Bax/Bcl-2 ratio (35-fold), caspase-3 (5-fold), and caspase-9 (4-fold) expression levels, when compared with the untreated group. | |
| BALB/c mice | 50 mg/kg | Neuroprotective activity [74] In LPS-stimulated mice: ~2-fold increase on the expression levels of PSD-95 and TH, when compared with untreated group. Reduction on expression levels of IL-1β (3-fold), IL-6 (2-fold), TNF-α (2-fold), and MCP-1 (2-fold), when compared with the untreated group. Reduction on the expression levels of COX-2 (3-fold) and iNOS (2-fold), when compared with the untreated group. Suppression of LPS-stimulated p38 (4-fold), ERK (4-fold), and JNK (2-fold) activation. | |
| Betulin (16) | C57BL/6 mice | 20 mg/kg | Hepatoprotective activity [75] Decrease of liver/body weight ratio in alcoholic mice, when compared with untreated group. Decrease in serum levels of ALT (66%), AST (33%), and TG (50%), when compared with the untreated group. Decrease on expression levels of collagen-I (20%), SREBP-1 (25%), and α-SMA (50%), when compared with untreated group. Increased phosphorylation of LKB1 (15%) and AMPK (25%) when compared with untreated group. 7-fold increase in SIRT-1 expression when compared with untreated group. |
| C57BL/6 mice | 50 mg/kg | Hepatoprotective activity [47] Decrease in serum levels of ALT (50%), AST (50%), and TG (33%) in ethanol induced fatty-liver mice, when compared with the untreated group. Decrease in expression levels of SREBP-1 (75%), CYP2E1 (60%), and TLR4 (60%), when compared with the untreated group. ~30% increase in phosphorylation of STAT3, when compared with the untreated group. |
| Name | Structure | Extract, Species, and Part of Plant |
|---|---|---|
| 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone (23) |  | Dichloromethane extract of Morella serrata (Lam.) Killick leaves [55] |
| Aurentiacin A (24) |  | Dichloromethane extract of Morella serrata (Lam.) Killick leaves [55] |
| 2′,6′-Dihydroxy-4′-methoxy-3′-methyl-dihydrochalcone (25) |  | Dichloromethane extract of Morella serrata (Lam.) Killick leaves [55] |
| Myrigalone E (26) |  | Methanol extract of Myrica gale L. seeds [54] From the Myrica gale L. fruits [53] Dichloromethane extract of Morella serrata (Lam.) Killick leaves [55] |
| Myrigalone P (27) |  | Methanol extract of Myrica gale L. seeds [54] |
| Uvangoletin (28) |  | Methanol extract of Myrica gale L. seeds [54] |
| Angoletin (29) |  | Fruit exudate from the Myrica gale L. fruits [53,56] |
| Demethoxymatteucinol (30) |  | Dichloromethane extract of Morella serrata (Lam.) Killick leaves [55] Methanol extract of Myrica gale L. seeds [54] |
| Cryptostrobin (31) |  | Dichloromethane extract of Morella serrata (Lam.) Killick leaves [55] |
| Demethoximatteucinol-7-methoxy (32) |  | Methanol extract of Myrica gale L. seeds [54] |
| Myricetin 3-O-6″-galloyl-β-D-galactopyranoside (33) |  | Methanol extract of Myrica gale L. (Myrica gale var tormentosa L.) branches [24] |
| Gallocatechin-4-α-8-epicatechin (34) |  | Aerial parts of Myrica gale L. [80] |
| Gallocatechin-4-α-8-epigallocatechin (35) |  | Aerial parts of Myrica gale L. [80] |
| Adenodimerin B (36) |  | Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] |
| Adenodimerin C (37) |  | Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] |
| Gallocatechin-(4-α-8)-gallocatechin-(4-α-8)-gallocatechin (38) |  | Aerial parts of Myrica gale L. [80] |
| Myricananin B (39) |  | Ethanol 80% extracts of Morella nana (A. Chev.) J. Herb. roots [28] |
| Myricananin E (40) |  | Ethanol 80% extracts of Morella nana (A. Chev.) J. Herb. roots [28] |
| Myricananin F (41) * |  | 80% ethanol extracts of Morella nana (A. Chev.) J. Herb. roots [81] |
| Myricananin G (42) |  | 80% ethanol extracts of the of Morella nana (A. Chev.) J. Herb. roots [81] |
| Myricananin H (43) |  | 80% ethanol extracts of Morella nana (A. Chev.) J. Herb. roots [81] |
| Myricatomentogenin (44) |  | Ethanol 80% extracts of Morella nana (A. Chev.) J. Herb. roots [81] |
| Myricanone 5-O-β-D-glucopyranoside (45) |  | Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] 80% ethanol extract of Morella nana (A. Chev.) J. Herb. roots [81] |
| Acerogenin 2-methyl ether (46) |  | 80% ethanol extract of Morella nana A. Chev.) J. Herb. roots [81] |
| Myricananone (47) |  | 95% EtOH extract of Morella nana A. Chev.) J. Herb. roots [21] |
| Myricananadiol (48) |  | 95% EtOH extract of Morella nana A. Chev.) J. Herb. roots [21] |
| Myricanol 11-O-β-D-xylopyranosyl (49) |  | Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] Dichloromethane: methanol (1:1) extract in of Morella arborea (Hutch.) Cheek bark and stem [29] |
| Myricatomentoside I (50) |  | Methanol extract of Myrica gale L. (Myrica gale var tormentosa L.) branches [35] |
| Myricatomentoside II (51) |  | Methanol extract of Myrica gale L. (Myrica gale var tormentosa L.) branches [35] |
| 12-Dehydroporson (52) |  | Methanol extract of Myrica gale L. (Myrica gale var tormentosa L.) branches [24] |
| Myricarborin (53) |  | Dichloromethane: methanol (1:1) extract of Morella arborea (Hutch.) Cheek bark and stem [29] |
| Myricanene A 5-O-α-L-arabinofuranosyl (1→6)-β-D-glucopyranoside (54) |  | Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] |
| Myresculoside (55) |  | Methanol extract of Myrica esculenta Buch.-Ham. ex D. Don leaves [14] |
| (1S,2S,4R)-2-Hydroxy-1,8-cineole β-D-glucopyranoside (56) |  | Methanol extract of Myrica esculenta Buch.-Ham. ex D. Don leaves [14] |
| Taraxerol (57) |  | Methanol extract of Morella adenophora (Hance) J. Herb. roots [19] Chloroform and methanol extract of Morella arborea (Hutch.) Cheek twigs [20] Hexane extract of Morella cerifera (L.) Small twigs [23] Benzene extract of Myrica gale L. (Myrica gale var tormentosa L.) stems [82] Benzene extract of Morella cerifera (L.) Small (Myrica cerifera) root bark [42] Extract of the bark of Myrica esculenta Buch.-Ham. ex D. Don [83] |
| Taraxerone (58) |  | Chloroform and methanol extract of Morella arborea (Hutch.) Cheek twigs [20] Benzene extract of Morella cerifera (L.) Small (Myrica cerifera) root bark [42] |
| Myricadiol (59) |  | Chloroform and methanol extract of Morella arborea (Hutch.) Cheek twigs [20] Benzene extract of Myrica gale L. (Myrica gale var tormentosa L.) stems [82] Hexane extract of Morella cerifera (L.) Small twigs [23] |
| Alphitolic acid (60) |  | Chloroform and methanol extract of Morella arborea (Hutch.) Cheek twigs [20] |
| Maslinic acid (61) |  | Chloroform and methanol extract of Morella arborea (Hutch.) Cheek twigs [20] |
| Myriceric acid C (62) |  | Methanol extract of Morella cerifera (L.) Small (Myrica cerifera) twigs [60] |
| Myriceric acid B (63) |  | Methanol extract of Morella cerifera (L.) Small (Myrica cerifera) twigs [60] |
| Myriceric acid D (64) |  | Methanol extract of Morella cerifera (L.) Small (Myrica cerifera) twigs [60] |
| Serratenedione (65) |  | Benzene extract of Myrica gale L. (Myrica gale var tormentosa L.) stems [82] |
| Serratenediol (66) |  | Benzene extract of Myrica gale L. (Myrica gale var tormentosa L.) stems [82] |
| Myricolal (67) |  | Benzene extract of Myrica gale L. (Myrica gale var tormentosa L.) stems [82] |
| Arjunglucoside (68) |  | Methanol extract of Myrica esculenta Buch.-Ham. ex D. Don leaves [14] |
| 3-epi-Ursolic acid (69) ** |  | Methanol extract of Myrica esculenta Buch.-Ham. ex D. Don leaves [14] |
| 3-O-(E)-Caffeoyl-ursolic acid (70) ** |  | Methanol extract of Myrica esculenta Buch.-Ham. ex D. Don leaves [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, G.P.; Silva, B.J.C.; Seca, A.M.L.; Moujir, L.M.; Barreto, M.C. Phytochemicals with Added Value from Morella and Myrica Species. Molecules 2020, 25, 6052. https://doi.org/10.3390/molecules25246052
Rosa GP, Silva BJC, Seca AML, Moujir LM, Barreto MC. Phytochemicals with Added Value from Morella and Myrica Species. Molecules. 2020; 25(24):6052. https://doi.org/10.3390/molecules25246052
Chicago/Turabian StyleRosa, Gonçalo P., Bruno J. C. Silva, Ana M. L. Seca, Laila M. Moujir, and Maria Carmo Barreto. 2020. "Phytochemicals with Added Value from Morella and Myrica Species" Molecules 25, no. 24: 6052. https://doi.org/10.3390/molecules25246052