Calcium Doped Flash-PEO Coatings for Corrosion Protection of Mg Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. PEO and CC Coatings Fabrication
2.2. Coating Characterization and Corrosion
3. Results and Discussion
3.1. Electrical Response of Flash-PEO
3.2. Coating Morphology and Composition
3.3. Electrochemical Impedance Response
- (i)
- PEO coatings enhance the corrosion performance of the bulk material for both immersion times showing higher values of the total impedance modulus up to two orders of magnitude for early immersion time.
- (ii)
- A considerable decrease in the impedance modulus is observed after 24 h of immersion for the coated specimens. This reduction of protective properties is associated with the penetration of the electrolyte through the cracks and pores of the oxide layer, leading to its chemical degradation.
- (iii)
- FPEO–CaGlyP coating reveals superior anticorrosion properties among the PEO coatings followed by FPEO–CaO and FPEO.
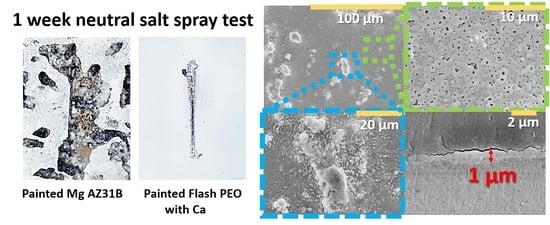
3.4. NSST and Paint Adhesion Test
4. Conclusions
- DC flash-PEO 1 µm-thick coatings were developed from dilute alkaline electrolytes without and with added Ca species stabilized in the solution with glycerophosphate and EDTA chelating agents.
- Chelating agents delayed the onset of plasma microdischarges and the attainment of the set limiting voltage, which reduced the coating porosity but did not significantly alter the coating thickness.
- The coating obtained from the electrolyte doped with Ca in a highly soluble form of CaGlyP disclosed one and two orders of magnitude improvement in corrosion protection over 24 h of immersion in 0.5 wt% NaCl compared with the coating generated from the CaO-doped and Ca-free electrolytes, respectively.
- All three flash-PEO coatings exhibited a rating of 0 in a cross-cut dry and wait paint adhesion test.
- Full system evaluation of the coatings in NSST for 7 days yielded ASTM D 1654 ratings 7 and 5 for FPEO_CaGlyP and commercial CC coatings, respectively.
- The enhanced corrosion resistance of FPEO–CaGlyP coating is attributed to the greater formation of covalent Si–O and P–O and ionic Ca–O bonds within the MgO ceramic network, which may have promoted the sealing of the flash-PEO coating pores with the hydrolysis products of SiO2.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Makar, G.L.; Kruger, J. Corrosion of Magnesium. Int. Mater. Rev. 1993, 38, 138–153. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Skeldon, P.; Thompson, G.E.; Pardo, A. Transport of Species during Plasma Electrolytic Oxidation of WE43-T6 Magnesium Alloy. J. Electrochem. Soc. 2008, 155, C101. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Ding, X.; Jiang, B.; Atrens, A.; Pan, F. Smart epoxy coating containing zeolites loaded with Ce on a plasma electrolytic oxidation coating on Mg alloy AZ31 for active corrosion protection. Prog. Org. Coat. 2019, 132, 144–147. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Ma, Y.; Song, G.; Zheng, D.; Jiang, B.; Atrens, A.; Pan, F. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corros. Sci. 2018, 139, 370–382. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, P.B.; Liang, J.; Blawert, C.; Stormer, M.; Dietzel, W. Characterization of calcium containing plasma electrolytic oxidation coatings on AM50 magnesium alloy. Appl. Surf. Sci. 2010, 256, 4017–4022. [Google Scholar] [CrossRef] [Green Version]
- Dou, J.; Zhao, Y.; Lu, L.; Gu, G.; Yu, H.; Chen, C. Effect of the Second-Step Voltages on the Structural and Corrosion Properties of Silicon-Calcium-Phosphate ( Si-CaP ) Coatings on Mg–Zn–Ca Alloy. R. Soc. Open Sci. 2018, 5, 172410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordbar-Khiabani, A.; Ghanbari, A.; Yarmand, B.; Zamanian, A.; Mozafari, M. Improving corrosion behavior and in vitro bioactivity of plasma electrolytic oxidized AZ91 magnesium alloy using calcium fluoride containing electrolyte. Mater. Lett. 2018, 212, 98–102. [Google Scholar] [CrossRef]
- Tang, H.; Gao, Y. Preparation and characterization of hydroxyapatite containing coating on AZ31 magnesium alloy by micro-arc oxidation. J. Alloys Compd. 2016, 688, 699–708. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Y.; Liu, L.; Liu, S.; Hou, B.; Liu, Q.; Ding, H. Effect of Ultrasonic Cold Forging Technology as the Pretreatment on the Corrosion Resistance of MAO Ca/P Coating on AZ31B Mg Alloy. J. Alloys Compd. 2015, 635, 278–288. [Google Scholar] [CrossRef]
- Pan, Y.K.; Chen, C.Z.; Wang, D.G.; Zhao, T.G. Colloids and Surfaces B: Biointerfaces Effects of Phosphates on Microstructure and Bioactivity of Micro-Arc Oxidized Calcium Phosphate Coatings on Mg–Zn–Zr Magnesium Alloy. Colloids Surf. B Biointerfaces 2013, 109, 1–9. [Google Scholar] [CrossRef]
- Gao, Y.; Yerokhin, A.; Parfenov, E.V.; Matthews, A. Application of Voltage Pulse Transient Analysis during Plasma Electrolytic Oxidation for Assessment of Characteristics and Corrosion Behaviour of Ca- and P-containing Coatings on Magnesium. Electrochimica. Acta 2014, 149, 218–230. [Google Scholar] [CrossRef]
- Yang, J.; Lu, X.; Blawert, C.; Di, S.; Zheludkevich, M.L. Microstructure and Corrosion Behavior of Ca/P Coatings Prepared on Magnesium by Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2017, 319, 359–369. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, C.; Wang, D.; Lin, Z. Preparation and bioactivity of micro-arc oxidized calcium phosphate coatings. Mater. Chem. Phys. 2013, 141, 842–849. [Google Scholar] [CrossRef]
- Pan, Y.K.; Chen, C.Z.; Wang, D.; Yu, X. Microstructure and biological properties of micro-arc oxidation coatings on ZK60 magnesium alloy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1574–1586. [Google Scholar] [CrossRef]
- Srinivasan, P.B.; Liang, J.; Blawert, C.; Stormer, M.; Dietzel, W. A preliminary study of calcium containing plasma electrolytic oxidation coatings on AM50 magnesium alloy. J. Mater. Sci. 2009, 45, 1406–1410. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Yerokhin, A.; Matthews, A. Effect of current mode on PEO treatment of magnesium in Ca- and P-containing electrolyte and resulting coatings. Appl. Surf. Sci. 2014, 316, 558–567. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Gnedenkov, S.; Khrisanfova, O.A.; Zavidnaya, A.G.; Egorkin, V.; Puz’, A.V.; Sergienko, V.I. Formation of bioactive anticorrosion coatings on resorbable implants by plasma electrolytic oxidation. Prot. Met. Phys. Chem. Surf. 2013, 49, 874–879. [Google Scholar] [CrossRef]
- Kuo, M.; Yen, S. The process of electrochemical deposited hydroxyapatite coatings on biomedical titanium at room temperature. Mater. Sci. Eng. C 2002, 20, 153–160. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, R.; Liu, C.; Gao, J. Comparison of calcium phosphate coatings on Mg–Al and Mg–Ca alloys and their corrosion behavior in Hank’s solution. Surf. Coat. Technol. 2010, 204, 3636–3640. [Google Scholar] [CrossRef]
- Wen, C.; Guan, S.-K.; Peng, L.; Ren, C.; Wang, X.; Hu, Z. Characterization and degradation behavior of AZ31 alloy surface modified by bone-like hydroxyapatite for implant applications. Appl. Surf. Sci. 2009, 255, 6433–6438. [Google Scholar] [CrossRef]
- Goss, S.L.; Lemons, K.A.; Kerstetter, J.E.; Bogner, R.H. Determination of calcium salt solubility with changes in pH and PCO2, simulating varying gastrointestinal environments. J. Pharm. Pharmacol. 2007, 59, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Zhang, F.; Lan, Z.; Cui, H.; Han, E. Corrosion Resistance of Calcium-Modified Zinc Phosphate Conversion Coatings on Magnesium—Aluminium Alloys. Corros. Sci. 2014, 88, 452–459. [Google Scholar] [CrossRef]
- Sreekanth, D.; Rameshbabu, N. Development and Characterization of MgO/Hydroxyapatite Composite Coating on AZ31 Magnesium Alloy by Plasma Electrolytic Oxidation Coupled with Electrophoretic Deposition. Mater. Lett. 2012, 68, 439–442. [Google Scholar] [CrossRef]
- Chang, L.; Tian, L.; Liu, W.; Duan, X. Corrosion Sc Ience Formation of Dicalcium Phosphate Dihydrate on Magnesium Alloy by Micro-Arc Oxidation Coupled with Hydrothermal Treatment. Corros. Sci. 2013, 72, 118–124. [Google Scholar] [CrossRef]
- Dorozhkin, S. Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta Biomater. 2014, 10, 2919–2934. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.B.; Witt, J.C. Solubility of Calcium Hydroxide. J. Phys. Chem. 1929, 33, 285–289. [Google Scholar] [CrossRef]
- Laleh, M.; Kargar, F.; Aghdam, A.S.R. Formation of a compact oxide layer on AZ91D magnesium alloy by microarc oxidation via addition of cerium chloride into the MAO electrolyte. J. Coat. Technol. Res. 2011, 8, 765–771. [Google Scholar] [CrossRef]
- Barchiche, C.-E.; Rocca, E.; Hazan, J. Corrosion behaviour of Sn-containing oxide layer on AZ91D alloy formed by plasma electrolytic oxidation. Surf. Coat. Technol. 2008, 202, 4145–4152. [Google Scholar] [CrossRef]
- Pezzato, L.; Brunelli, K.; Napolitani, E.; Magrini, M.; Dabalà, M. Surface properties of AZ91 magnesium alloy after PEO treatment using molybdate salts and low current densities. Appl. Surf. Sci. 2015, 357, 1031–1039. [Google Scholar] [CrossRef]
- Zhao, F.; Liao, A.-D.; Zhang, R.; Zhang, S.-F.; Wang, H.-X.; Shi, X.-M.; Li, M.-J.; He, X.-M. Effects of sodium tungstate on properties of micro-arc coatings on magnesium alloys. Trans. Nonferrous Met. Soc. China 2010, 20, s683–s687. [Google Scholar] [CrossRef]
- Cui, X.-J.; Liu, C.-H.; Yang, R.; Li, M.-T.; Lin, X.-Z. Self-sealing micro-arc oxidation coating on AZ91D Mg alloy and its formation mechanism. Surf. Coat. Technol. 2015, 269, 228–237. [Google Scholar] [CrossRef]
- Hwang, D.Y.; Cho, J.Y.; Lee, N.H.; Yoo, B.Y.; Shin, D.H. Plasma Electrolytic Oxidation of AZ91 Mg Alloy in the Sodium Stannate Electrolyte. Mater. Trans. 2008, 49, 1600–1605. [Google Scholar] [CrossRef] [Green Version]
- Ko, Y.; Namgung, S.; Shin, D.H. Correlation between KOH concentration and surface properties of AZ91 magnesium alloy coated by plasma electrolytic oxidation. Surf. Coat. Technol. 2010, 205, 2525–2531. [Google Scholar] [CrossRef]
- Ghasemi, A.; Raja, V.; Blawert, C.; Dietzel, W.; Kainer, K. The role of anions in the formation and corrosion resistance of the plasma electrolytic oxidation coatings. Surf. Coat. Technol. 2010, 204, 1469–1478. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, P.B.; Liang, J.; Balajeee, R.; Blawert, C.; Stormer, M.; Dietzel, W. Effect of pulse frequency on the microstructure, phase composition and corrosion performance of a phosphate-based plasma electrolytic oxidation coated AM50 magnesium alloy. Appl. Surf. Sci. 2010, 256, 3928–3935. [Google Scholar] [CrossRef] [Green Version]
- Clyne, T.; Troughton, S. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2018, 64, 127–162. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Sah, S.P.; Scharnagl, N.; Störmer, M.; Starykevich, M.; Mohedano, M.; Blawert, C.; Zheludkevich, M.L.; Kainer, K.U. Degradation behavior of PEO coating on AM50 magnesium alloy produced from electrolytes with clay particle addition. Surf. Coat. Technol. 2015, 269, 155–169. [Google Scholar] [CrossRef]
- Lee, S.; Ueda, K.; Narushima, T.; Nakano, T.; Kasuga, T. Preparation of orthophosphate glasses in the MgO–CaO–SiO2–Nb2O5–P2O5 system. Bio-Med. Mater. Eng. 2017, 28, 23–30. [Google Scholar] [CrossRef]
- Karakassides, M.; Saranti, A.; Koutselas, I. Preparation and structural study of binary phosphate glasses with high calcium and/or magnesium content. J. Non-Cryst. Solids 2004, 347, 69–79. [Google Scholar] [CrossRef]
- Lee, S.; Nakano, T.; Kasuga, T. Formation and Structural Analysis of 15MgO–15CaO–8P2O5–4SiO2 Glass. J. Non-Cryst. Solids 2017, 457, 73–76. [Google Scholar] [CrossRef]
- Cheng, J.-S.; Deng, W.; Wang, M. Structure of Na2O·MO·SiO2·CaF2 (M=Mg, Ca) oxyfluoride glasses. Phys. B Condens. Matter 2012, 407, 2778–2783. [Google Scholar] [CrossRef]
- Kirkpatrick, R.J.; Yarger, J.; McMillan, P.F.; Ping, Y.; Cong, X. Raman spectroscopy of C-S-H, tobermorite, and jennite. Adv. Cem. Based Mater. 1997, 5, 93–99. [Google Scholar] [CrossRef]
- Agathopoulos, S.; Tulyaganov, D.; Ventura, J.M.; Kannan, S.; Karakassides, M.; Ferreira, J.M.F. Formation of hydroxyapatite onto glasses of the CaO–MgO–SiO2 system with B2O3, Na2O, CaF2 and P2O5 additives. Biomaterials 2006, 27, 1832–1840. [Google Scholar] [CrossRef]
- De Aza, P.N.; Guitian, F.; Santos, C.; De Aza, S.; Cuscó, R.; Artús, L. Vibrational Properties of Calcium Phosphate Compounds. 2. Comparison between Hydroxyapatite and β-Tricalcium Phosphate. Chem. Mater. 1997, 9, 916–922. [Google Scholar] [CrossRef]
- Huelin, S.D.; Baker, H.R.; Merschrod, S.E.F.; Poduska, K.M. Phase-Selective Electroprecipitation of Calcium Phosphate Thin Films at Physiological Temperatures. Cryst. Growth Des. 2006, 6, 2634–2636. [Google Scholar] [CrossRef]
- Iqbal, Z.; Tomaselli, V.P.; Fahrenfeld, O.; Möller, K.D.; Ruszala, F.A.; Kostiner, E. Polarized Raman Scattering and Low Frequency Infrared Study of Hydroxyapatite. J. Phys. Chem. Solids 1977, 38, 923–927. [Google Scholar] [CrossRef]
- Agathopoulos, S.; Tulyaganov, D.; Ventura, J.; Kannan, S.; Saranti, A.; Karakassides, M.; Ferreira, J.M.F. Structural analysis and devitrification of glasses based on the CaO–MgO–SiO2 system with B2O3, Na2O, CaF2 and P2O5 additives. J. Non-Cryst. Solids 2006, 352, 322–328. [Google Scholar] [CrossRef]
- Yu, H.; Dong, Q.; Dou, J.; Pan, Y.; Chen, C.Z. Structure and in vitro bioactivity of ceramic coatings on magnesium alloys by microarc oxidation. Appl. Surf. Sci. 2016, 388, 114–119. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, M.; Yang, X.; Huang, P.; Xu, K. Effect of Na 2 SiO 3 solution concentration of micro-arc oxidation process on lap-shear strength of adhesive-bonded magnesium alloys. Appl. Surf. Sci. 2014, 314, 447–452. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Gao, S.-D.; Li, P.-P.; Zeng, R.; Zhang, F.; Zeng, R.-C.; Han, E.-H. Corrosion resistance of a self-healing micro-arc oxidation/polymethyltrimethoxysilane composite coating on magnesium alloy AZ31. Corros. Sci. 2017, 118, 84–95. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Li, J.; Li, Y.; Lu, S.; Yuan, Y. The enhanced corrosion resistance of UMAO coatings on Mg by silane treatment. Prog. Nat. Sci. 2014, 24, 486–491. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, H.; Solla, E.; Serra, J.; González, P.; León, B.; Almeida, N.; Cachinho, S.; Davim, E.; Correia, R.; Oliveira, J.; et al. Orthophosphate nanostructures in SiO2–P2O5–CaO–Na2O–MgO bioactive glasses. J. Non-Cryst. Solids 2008, 354, 4075–4080. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.; Hamzah, E.; Daroonparvar, M.; Yajid, M.; Medraj, M. Fabrication and corrosion behavior of Si/HA nano-composite coatings on biodegradable Mg–Zn–Mn–Ca alloy. Surf. Coat. Technol. 2014, 258, 1090–1099. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Pardo, A.; Merino, M.C.; Paucar, K.; Mohedano, M.; Casajús, P. Corrosion behaviour of AZ91D and AM50 magnesium alloys with Nd and Gd additions in humid environments. Corros. Sci. 2012, 55, 351–362. [Google Scholar] [CrossRef]
- Baril, G.; Galicia, G.; Deslouis, C.; Pébère, N.; Tribollet, B.; Vivier, V. An Impedance Investigation of the Mechanism of Pure Magnesium Corrosion in Sodium Sulfate Solutions. J. Electrochem. Soc. 2007, 154, C108–C113. [Google Scholar] [CrossRef] [Green Version]
- King, A.; Birbilis, N.; Scully, J. Accurate Electrochemical Measurement of Magnesium Corrosion Rates; a Combined Impedance, Mass-Loss and Hydrogen Collection Study. Electrochim. Acta 2014, 121, 394–406. [Google Scholar] [CrossRef]
- Giju, K.T. Effect of coordinated water on the mechanism of neutral hydrolysis of silicon dioxide in gas phase: A first principles study. J. Mol. Struct. THEOCHEM 2002, 592, 53–60. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Ismail, A.; Aziz, M.; Najafinezhad, A.; Daroonparvar, M. Synthesis and in-vitro performance of nanostructured monticellite coating on magnesium alloy for biomedical applications. J. Alloys Compd. 2019, 773, 180–193. [Google Scholar] [CrossRef]











| Nº | Alloy/Source of Ca in Electrolyte | PEO Parameters | Coating Composition | Substrate/ Coating Thickness (µm) | Corrosion Data | Ref. | |
|---|---|---|---|---|---|---|---|
| Ecorr (V) | icorr (A/cm2) | ||||||
| 1 | AM50 | 30 mA/cm2, pulse on: 2 ms, off: 18 ms, Vmax 485 V, 15 min | MgO, Mg3(PO4)2, CaH(PO4)2, CaO2 | 0 | −1.452 | 1.8 × 10−5 | [5] |
| Ca(OH)2 | 18–23 | −1.52 | 2.3 × 10−7 | ||||
| 35–44 | −1.505 | 3.5 × 10−8 | |||||
| 57–69 | −1.495 | 5.2 × 10−8 | |||||
| 2 | Mg + Zn 1.74, Ca 0.55 wt %x 1st step: Ca-free 2nd step: (C3H7O6P)Ca | 600 Hz, positive/negative duty ratio 30/20%, C1: 400 V 5 min C2: 400 V C3: 450 V C4: 500 V 5 min | C1, C2: MgF2, MgSiO3, SiO2,Mg2SiO4 C3, C4: MgF2, MgSiO3, SiO2, Mg2SiO4, Ca3(PO4)2 | 0 | −1.731 | 4.29 × 10−4 | [6] |
| C1: 20 | −1.725 | 1.78 × 10−6 | |||||
| C2: 40 | −1.721 | 1.62 × 10−7 | |||||
| C3: 70–90 | −1.429 | 6.31 × 10−8 | |||||
| C4: 210–250 | −1.708 | 3.01 × 10−7 | |||||
| 3 | AZ91 CaF2 | 100 mA/cm2, duty cycle 25%, | - | 0 | −1.733 | 126.71 × 10−6 | [7] |
| ? | −1.609 | 98.66 × 10−6 | |||||
| ? | −1.348 | 0.93 × 10−6 | |||||
| 4 | AZ31 | 400 V/0 V, 100 Hz 10 min | MgO, HA | 0 | −1.47 | 1.05 × 10−5 | [8] |
| CaGlyP, NaOH (varied) | 7 | −1.01 | 3.62 × 10−6 | ||||
| 9.8 | −0.79 | 9.83 × 10−7 | |||||
| 14.7 | −0.72 | 1.27 × 10−6 | |||||
| 19.8 | −0.65 | 3.02 × 10−6 | |||||
| 5 | Mg (purity 99%) | 30 mA/cm2. Unipolar, 100 to 5000 Hz, duty cycle 10%, 10 min | MgO, Na4Ca(PO3)6. | >20 | −1.6 to −1.4 | - | [11] |
| Ca(OH)2 | |||||||
| 6 | hp-Mg | DC 50 mA/cm2, 200 Hz, duty cycle, 20%, 10 min | Mg3(PO4)2, MgO, HA, Na2CaMg7(PO4)6 | 43.9 ± 4.8 36.8 ± 1.2 36.8 ± 3.4 33.1 ± 2.7 | Improved corrosion resistance for Ca presence (EIS) | [12] | |
| HA | |||||||
| 7 | ZK60 | positive/negative 450/20V, 600 Hz, positive/negative duty ratio 30/20%, positive : Negative pulses 1:1, 30 min | MgO, MgF2, ZnF2, ZnO, CaO, CaF2, Ca3(PO4)2 | 0 | −1.5 | 7.65 × 10−5 | [14] |
| (C6H5O7)2Ca3∙4H2O | ~35–70 | −1.521 | 2.74 × 10−9 | ||||
| −1.508 | 2.17 × 10−6 | ||||||
| −1.377 | 5.57 × 10−6 | ||||||
| 8 | AM50 | DC 30 mA/cm2, ton:toff = 2:20 ms, 15 min | MgO and Mg3(PO3)2 + amorphous CaH(PO4), CaO | corrosion resistance (EIS) | [15] | ||
| Ca(OH)2 | 56 ± 6 | 6 × 105 Ω cm2 (0.5 h) 5 × 104 Ω cm2 (5–10 h) 5 × 103 Ω cm2 (50 h) | |||||
| 38 ± 5 | 8 × 105 Ω cm2 (0.5 h) 4 × 105 Ω cm2 (5 h) 1.5 × 105 Ω cm2 (50 h) 6 × 104 Ω cm2 (150 h) | ||||||
| 9 | Commercially pure | pulsing frequency 3000 Hz: positive 30, negative 0 to 20 mA/cm2 duty cycles 10%, 10 min | MgO, Na4Ca(PO3)6 | 0 | −1.56 | 4.38 × 10−4 | [16] |
| Ca(OH)2 | Unipolar | −1.41 | 1. 45 × 10−5 | ||||
| Bipolar | −1.43 | 1.81 × 10−5 | |||||
| 10 | MA8 | Bipolar mode | MgO, Ca10(PO4)6(OH)2 | 0 | −1.564 | 5.10 × 10−5 | [17] |
| (C3H7O6P)Ca | - | −1.519 | 1.15 × 10−6 | ||||
| Nº | Alloy/Electrolyte | PEO Parameters | Coating Composition | Substrate/Coating Thickness (µm) | Corrosion Data | Ref | ||
|---|---|---|---|---|---|---|---|---|
| Ecorr (V) | icorr (A/cm2) | R Inner (Ω cm2) | ||||||
| 1 | AZ91D | - | - | 0 | −1.509 | 2.352 × 10−6 | - | [27] |
| NaAlO2, KOH | 25 mA/cm2, 25 min | - | 5 | −1.479 | 1.607 × 10−6 | 1866 | ||
| + CeCl3 | 5 | −1.607 | 0.179 × 10−6 | 52547 | ||||
| 2 | AZ91D | 0 | −1.4 | - | - | [28] | ||
| KOH, KF, Na3PO4, K2SnO3 | 10 mA/cm2, 10 min | MgSn(OH)6, amorphous MgO | 5–10 | −1.55 to −1.6 | - | |||
| 3 | AZ91 Na2SiO3, NaOH, diethyl-amine, Na2MoO4 | - | - | 0 | −1.55 | 1.5 × 10−5 | 1259 | [29] |
| 50 mA/cm2, 15 min | MgO, Mg2SiO4, | 40 | −1.39 | 3.5 × 10−5 | 800 | |||
| MoO3, MgMoO4 | 1 | −1.37 | 1 × 10−6 | 42721 | ||||
| 4 | AZ91HP NaOH, phytic acid, Na2WO4 | 0 | - | - | - | [30] | ||
| 40 mA/cm2, 2000 Hz | - | 4.9 | one small corrosion pit | |||||
| 4.6 | four corrosion pits, largest pit 8 mm × 6 mm | |||||||
| 5 | AZ91D Na2SiO3, NaF, NH4H2PO4, C6H5O7Na3, K2ZrF6 | 0 | - | - | - | |||
| 300 V, 480 Hz, duty cycle 30%, 45 °C, 10 min | MgO, MgF2, MgSiO3, amorphous phosphate | 5–8 | −1.503 | 6.57 × 10−6 | 1,290,000 | [31] | ||
| t-ZrO2 | −1.421 | 4.04 × 10−7 | 1,064,0000 | |||||
| 6 | AZ91 | 0 | - | - | - | [32] | ||
| KOH, KF, Na2SiO3 Na2SnO3 | 20 mA/cm2, 2–10 min | MgO, SnO, Mg2SiO4, MgF2 | 1.5 | −1.55 | 3.07 × 10−7 | - | ||
| 5 | −1.39 | 1.93 × 10−10 | - | |||||
| 7 | AZ91 | 0 | - | - | - | [33] | ||
| Na2SiO3, KF, KOH | 25 mA/cm2, 5 min | MgO, MgOH2 | ~1.4 | −1.45 | 1.32 × 10−8 | 396,000 | ||
| ~1.4 | −1.34 | 2.46 × 10−9 | 465,000 | |||||
| 8 | AM50 KOH, Na2SiO4 KOH, Na3PO4 KOH, NaAlO2 | 0 | - | - | - | [34] | ||
| 36.2 A/cm2 pulse ratio tmax:tmin = 4 ms:1 ms, 50 Hz, 5 min | MgO, Mg2SiO4 | 8 ± 0.7 | - | 3.2 × 10−6 | 5,640,000 | |||
| MgO, Mg3(PO4)2 | 4 ± 0.4 | -- | 9.5 × 10−4 | 310,000 | ||||
| MgO, MgAl2O4 | 1 ± 0.2 | - | 8.7 × 10−3 | 2300 | ||||
| Name | Electrolyte | pH/Conductivity (mS/cm) | Treatment Time (s) | Energy Consumption (kWh/m2) | Pore Population Density/Pore Diameter (µm−2)/(µm) |
|---|---|---|---|---|---|
| FPEO | 5 g/L Na2SiO3·5H2O, 5 g/L Na3PO4·12H2O, 14 g/L KOH, 3 g/L KF | 12.91/57.5 | 20 | 0.80 | 3.1 ± 0.5/(14 ± 2) × 10−3 |
| FPEO_CaO | 5 g/L Na2SiO3·5H2O, 5 g/L Na3PO4·12H2O, 14 g/L KOH, 3 g/L KF, 2 mM Na2EDTA, 1.5 mM CaO | 12.94/59.2 | 35 | 1.64 | 1.8 ± 0.3/(8 ± 3) × 10−3 |
| FPEO_CaGlyP | 5 g/L Na2SiO3·5H2O, 5 g/L Na3PO4·12H2O, 14 g/L KOH, 3 g/L KF, 2 mM Na2EDTA, 1.5 mM CaGlyP | 12.91/57.8 | 45 | 2.04 | 1.9 ± 0.8/(10 ± 4) × 10−3 |
| Elements | FPEO | FPEO_CaO | FPEO_CaGlyP |
|---|---|---|---|
| Mg | 56.1 ± 1.2 | 50.6 ± 0.4 | 52.4 ± 1.1 |
| Al | 2.9 ± 0.5 | 3.2 ± 0.5 | 2.5 ± 0.4 |
| O | 36.4 ± 1.1 | 39.6 ± 0.3 | 39.6 ± 0.7 |
| F | 2.1 ± 0.4 | 3.5 ± 0.5 | 2.6 ± 0.5 |
| Si | 1.8 ± 0.1 | 2.3 ± 0.06 | 2.0 ± 0.2 |
| P | 0.7 ± 0.2 | 0.9 ± 0.03 | 0.7 ± 0.2 |
| Ca | - | 0.1 ± 0.04 | 0.1 ± 0.04 |
| Sample | Re | CPE1 | n | R1 | CPE2 | n | R2 | L | R3 | Rt | Rp |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg | 142.3 | 8.37 × 10−6 | 0.87 | 320 | 1.7 × 10−6 | 0.90 | 856 | 836.22 | 603 | 1176 | 399 |
| Bonderite CC | 178.1 | 1.06 × 10−6 | 0.90 | 350 | 4.3 × 10−6 | 0.90 | 2400 | 2873 | 2060 | 2750 | 1178 |
| Sample | Re | CPEcp | n | Rcp | CPEdl | n | Rp |
|---|---|---|---|---|---|---|---|
| Mg | 152.2 | 1.41 × 10−5 | 0.90 | 120 | 6.37 × 10−6 | 0.90 | 1047 |
| Bonderite | 153.5 | 7.96 × 10−6 | 0.94 | 228 | 8.73 × 10−6 | 0.98 | 1173 |
| Sample | Re | CPEout | n | Rout | CPEin | n | Rin | CPEdl | n | Rp |
|---|---|---|---|---|---|---|---|---|---|---|
| FPEO | 140.7 | 4.5 × 10−7 | 0.83 | 70 | 1.46 × 10−6 | 0.60 | 1.25 × 105 | 2.30 × 10−4 | 0.97 | 0.37 × 105 |
| FPEO_CaO | 133.4 | 2.4 × 10−7 | 0.83 | 2553 | 1.85 × 10−7 | 0.86 | 4.02 × 105 | 2.23 × 10−5 | 0.71 | 2.73 × 105 |
| FPEO_CaGlyP | 161.8 | 1.65 × 10−7 | 0.84 | 2550 | 4.22 × 10−8 | 0.83 | 6.79 × 105 | 1.06 × 10−5 | 0.61 | 5.98 × 105 |
| Sample | Re | CPEcoat | n | Rcoat | CPEdl | n | Rp |
|---|---|---|---|---|---|---|---|
| FPEO | 154.1 | 1.74 × 10−6 | 0.77 | 2230 | 2.08 × 10−7 | 1 | 0.11 × 105 |
| FPEO_CaO | 172.1 | 1.54 × 10−7 | 0.94 | 8464 | 1.44 × 10−7 | 0.92 | 0.76 × 105 |
| FPEO_CaGlyP | 152.6 | 3.61 × 10−7 | 0.87 | 1.54 × 105 | 1.13 × 10−5 | 0.68 | 1.14 × 105 |
| Sample | Length of Corroded Point (mm) | Rating ASTM D 1654 | Corroded Surface Area (mm2) |
|---|---|---|---|
| Mg substrate | not applicable | 0 | 150 ± 41.11 |
| CC | 4.1 ± 0.14 | 5 | 11 ± 0.98 |
| FPEO | 2 ± 1.53 | 6 | 5 ± 2.1 |
| FPEO_CaO | 2.3 ± 0.49 | 6 | 4.1 ± 0.69 |
| FPEO_CaGlyP | 1.9 ± 0.28 | 7 | 2.2 ± 0.49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbicka, E.; Pillado, B.; Mohedano, M.; Arrabal, R.; Matykina, E. Calcium Doped Flash-PEO Coatings for Corrosion Protection of Mg Alloy. Metals 2020, 10, 916. https://doi.org/10.3390/met10070916
Wierzbicka E, Pillado B, Mohedano M, Arrabal R, Matykina E. Calcium Doped Flash-PEO Coatings for Corrosion Protection of Mg Alloy. Metals. 2020; 10(7):916. https://doi.org/10.3390/met10070916
Chicago/Turabian StyleWierzbicka, Ewa, Borja Pillado, Marta Mohedano, Raul Arrabal, and Endzhe Matykina. 2020. "Calcium Doped Flash-PEO Coatings for Corrosion Protection of Mg Alloy" Metals 10, no. 7: 916. https://doi.org/10.3390/met10070916