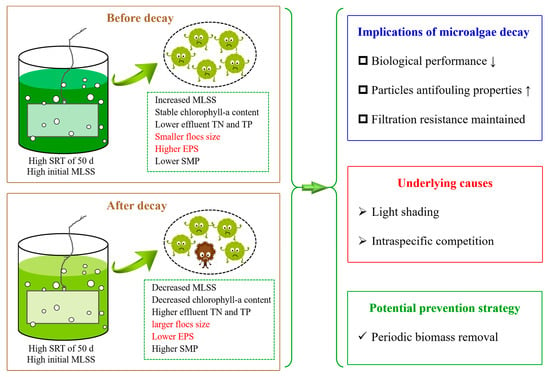
Membrane Photobioreactor Applied for Municipal Wastewater Treatment at a High Solids Retention Time: Effects of Microalgae Decay on Treatment Performance and Biomass Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. MPBR Setup and Operation
2.2. Extraction and Analysis of Chlorophyll-a
2.3. PSD Analysis and Microscopic Observation
2.4. Soluble Microbial Products (SMP) and Extracellular Polymeric Substances (EPS) Measurement
2.5. Other Analysis
3. Results
3.1. Biomass Concentration and Chlorophyll-a Content
3.2. Nutrients Removal
3.3. Microalgae Properties and Membrane Fouling
3.3.1. PSD and Micromorphology
3.3.2. EPS and SMP
3.3.3. Membrane Fouling Performance
3.4. Implications of High SRT for Long-Term Municipal Wastewater Treatment in MPBR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bouju, H.; Buttiglieri, G.; Malpei, F. The use of microcalorimetry to compare the biological activity of a CAS and a MBR sludge-application to pharmaceutical active compounds. Water Sci. Technol. 2008, 58, 529–535. [Google Scholar] [CrossRef]
- Munz, G.; Gualtiero, M.; Salvadori, L.; Claudia, B.; Claudio, L. Process efficiency and microbial monitoring in MBR (membrane bioreactor) and CASP (conventional activated sludge process) treatment of tannery wastewater. Bioresour. Technol. 2008, 99, 8559–8564. [Google Scholar] [CrossRef]
- Cicek, N. A review of membrane bioreactors and their potential application in the treatment of agricultural wastewater. Can. Biosyst. Eng. 2003, 45, 37–49. [Google Scholar]
- Lin, H.; Gao, W.; Meng, F.; Liao, B.-Q.; Leung, K.-T.; Zhao, L.; Chen, J.; Hong, H. Membrane bioreactors for industrial wastewater treatment: A critical review. Crit. Rev. Env. Sci. Technol. 2012, 42, 677–740. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef]
- Wu, M.; Chen, Y.; Lin, H.; Zhao, L.; Shen, L.; Li, R.; Xu, Y.; Hong, H.; He, Y. Membrane fouling caused by biological foams in a submerged membrane bioreactor: Mechanism insights. Water Res. 2020, 181, 115932. [Google Scholar] [CrossRef]
- Abinandan, S.; Shanthakumar, S. Challenges and opportunities in application of microalgae (Chlorophyta) for wastewater treatment: A review. Renew. Sust. Energ. Rev. 2015, 52, 123–132. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, L.; Qi, Y. Enhancing the productivity of microalgae cultivated in wastewater toward biofuel production: A critical review. Appl. Energy 2015, 137, 282–291. [Google Scholar] [CrossRef]
- Razzak, S.A.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; de Lasa, H. Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renew. Sust. Energ. Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Luo, Y.; Le-Clech, P.; Henderson, R.K. Simultaneous microalgae cultivation and wastewater treatment in submerged membrane photobioreactors: A review. Algal Res. 2017, 24, 425–437. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Z.-H.; Li, C.; Wang, Y.-J.; Jin, W.-H.; Deng, Y.-B. Concentrated microalgae cultivation in treated sewage by membrane photobioreactor operated in batch flow mode. Bioresour. Technol. 2014, 167, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Maity, J.P.; Bundschuh, J.; Chen, C.-Y.; Bhattacharya, P. Microalgae for third generation biofuel production, mitigation of greenhouse gas emissions and wastewater treatment: Present and future perspectives—A mini review. Energy 2014, 78, 104–113. [Google Scholar] [CrossRef]
- Marbelia, L.; Bilad, M.R.; Passaris, I.; Discart, V.; Vandamme, D.; Beuckels, A.; Muylaert, K.; Vankelecom, I.F.J. Membrane photobioreactors for integrated microalgae cultivation and nutrient remediation of membrane bioreactors effluent. Bioresour. Technol. 2014, 163, 228–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonchai, R.; Seo, G. Microalgae membrane photobioreactor for further removal of nitrogen and phosphorus from secondary sewage effluent. Korean, J. Chem. Eng. 2015, 32, 2047–2052. [Google Scholar] [CrossRef]
- Xu, M.; Li, P.; Tang, T.; Hu, Z. Roles of SRT and HRT of an algal membrane bioreactor system with a tanks-in-series configuration for secondary wastewater effluent polishing. Ecol. Eng. 2015, 85, 257–264. [Google Scholar] [CrossRef]
- Gao, F.; Li, C.; Yang, Z.-H.; Zeng, G.-M.; Feng, L.-J.; Liu, J.-Z.; Liu, M.; Cai, H.-W. Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecol. Eng. 2016, 92, 55–61. [Google Scholar] [CrossRef]
- Honda, R.; Teraoka, Y.; Noguchi, M.; Yang, S. Optimization of Hydraulic Retention Time and Biomass Concentration in Microalgae Biomass Production from Treated Sewage with a Membrane Photobioreactor. J. Water Environ. Technol. 2017, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Le-Clech, P.; Henderson, R.K. Assessment of membrane photobioreactor (MPBR) performance parameters and operating conditions. Water Res. 2018, 138, 169–180. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, L.; Maleki, E.; Liao, B.-Q.; Lin, H. Membrane technologies for microalgal cultivation and dewatering: Recent progress and challenges. Algal Res. 2019, 44, 101686. [Google Scholar] [CrossRef]
- Van Thuan, N.; Thi Thanh Thuy, N.; Hong Hai, N.; Nguyen, N.C.; Bui, X.-T. Influence of microalgae retention time on biomass production in membrane photobioreactor using human urine as substrate. Vietnam, J. Sci. Technol. Eng. 2018, 60, 5. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Leung, K.-T.; Lin, H.; Liao, B. Effects of solids retention time on the biological performance of a novel microalgal-bacterial membrane photobioreactor for industrial wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 105500. [Google Scholar] [CrossRef]
- Xu, M.; Bernards, M.; Hu, Z. Algae-facilitated chemical phosphorus removal during high-density Chlorella emersonii cultivation in a membrane bioreactor. Bioresour. Technol. 2014, 153, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Praveen, P.; Guo, Y.; Kang, H.; Lefebvre, C.; Loh, K.-C. Enhancing microalgae cultivation in anaerobic digestate through nitrification. Chem. Eng. J. 2018, 354, 905–912. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; He, N.; Zheng, Y.; Li, H.; Wang, H.; Wang, Y.; Lu, Y.; Li, Q.; Peng, Y. Nitrogen and phosphorus removal from anaerobically digested wastewater by microalgae cultured in a novel membrane photobioreactor. Biotechnol. Biofuels 2018, 11, 190. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Quan, X.; Zhong, N.; Zhang, Z.; Lu, C.; Li, G.; Cheng, Z.; Yang, L. High-efficiency nutrients reclamation from landfill leachate by microalgae Chlorella vulgaris in membrane photobioreactor for bio-lipid production. Bioresour. Technol. 2018, 266, 374–381. [Google Scholar] [CrossRef]
- Zhang, M.; Leung, K.-T.; Lin, H.; Liao, B. The biological performance of a novel microalgal-bacterial membrane photobioreactor: Effects of HRT and N/P ratio. Chemosphere 2020, 261, 128199. [Google Scholar] [CrossRef]
- Nautiyal, P.; Subramanian, K.A.; Dastidar, M.G. Production and characterization of biodiesel from algae. Fuel Process. Technol. 2014, 120, 79–88. [Google Scholar] [CrossRef]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Zhang, M.; Leung, K.-T.; Lin, H.; Liao, B. Membrane fouling in a microalgal-bacterial membrane photobioreactor: Effects of P-availability controlled by N:P ratio. Chemosphere 2021, 282, 131015. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Gaudy, A.F. Colorimetric determination of protein and carbohydrate. Ind. Water Wastes 1962, 7, 17–22. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association; American Water Works Association; Water Environmental Federation: Washington, DC, USA, 2005. [Google Scholar]
- Liu, J.; Wu, Y.; Wu, C.; Muylaert, K.; Vyverman, W.; Yu, H.-Q.; Muñoz, R.; Rittmann, B. Advanced nutrient removal from surface water by a consortium of attached microalgae and bacteria: A review. Bioresour. Technol. 2017, 241, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Guieysse, B. Algal–bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Ding, S.; Yao, L.; Shen, Q.; Zhu, C.; Wang, Y.; Xu, D. Dynamics of phosphorus–iron–sulfur at the sediment–water interface influenced by algae blooms decomposition. J. Hazard. Mater. 2015, 300, 329–337. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Wang, G.; Li, X.; Ma, J.; Chen, S.; Deng, H.; Annalisa, O.-H. High sulfide production induced by algae decomposition and its potential stimulation to phosphorus mobility in sediment. Sci. Total Environ. 2019, 650, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yao, Y.; Lian, J.; Ma, C. Effect of thermal stratified flow on algal blooms in a tributary bay of the Three Gorges reservoir. J. Hydrol. 2021, 601, 126648. [Google Scholar] [CrossRef]
- Yao, Y.; Li, D.; Chen, Y.; Han, X.; Wang, G.; Han, R. High-resolution characteristics and mechanisms of endogenous phosphorus migration and transformation impacted by algal blooms decomposition. Sci. Total Environ. 2022, 820, 152907. [Google Scholar] [CrossRef]
- Shen, L.-G.; Lei, Q.; Chen, J.-R.; Hong, H.-C.; He, Y.-M.; Lin, H.-J. Membrane fouling in a submerged membrane bioreactor: Impacts of floc size. Chem. Eng. J. 2015, 269, 328–334. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Fan, Y.; Yang, H.; Wang, Z.; Chen, Y.; Tang, C.Y. Dissect the role of particle size through collision-attachment simulations for colloidal fouling of RO/NF membranes. J. Membr. Sci. 2021, 638, 119679. [Google Scholar] [CrossRef]
- Zhang, M.; Hong, H.; Lin, H.; Shen, L.; Yu, H.; Ma, G.; Chen, J.; Liao, B.-Q. Mechanistic insights into alginate fouling caused by calcium ions based on terahertz time-domain spectra analyses and DFT calculations. Water Res. 2018, 129, 337–346. [Google Scholar] [CrossRef]
- Teng, J.; Zhang, M.; Leung, K.-T.; Chen, J.; Hong, H.; Lin, H.; Liao, B.-Q. A unified thermodynamic mechanism underlying fouling behaviors of soluble microbial products (SMPs) in a membrane bioreactor. Water Res. 2019, 149, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Siddharth, T.; Sridhar, P.; Vinila, V.; Tyagi, R.D. Environmental applications of microbial extracellular polymeric substance (EPS): A review. J. Environ. Manag. 2021, 287, 112307. [Google Scholar] [CrossRef] [PubMed]
- Martis, B.S.; Mohan, A.K.; Chiplunkar, S.; Kamath, S.; Goveas, L.C.; Rao, C.V. Bacterium isolated from coffee waste pulp biosorps lead: Investigation of EPS mediated mechanism. Curr. Res. Microb. Sci. 2021, 2, 100029. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, M.; Wang, F.; Meng, F.; Liao, B.-Q.; Hong, H.; Chen, J.; Gao, W. A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: Characteristics, roles in membrane fouling and control strategies. J. Membr. Sci. 2014, 460, 110–125. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Liu, T.; Zheng, X.; Tang, G.; Yang, X.; Zhi, H.; Qiu, X.; Li, X.; Wang, Z. Effects of temperature shocks on the formation and characteristics of soluble microbial products in an aerobic activated sludge system. Process Saf. Environ. Prot. 2022, 158, 231–241. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Z.; Ding, Y.; Lu, Y. Identification of the change in fouling potential of soluble microbial products (SMP) in membrane bioreactor coupled with worm reactor. Water Res. 2013, 47, 2015–2024. [Google Scholar] [CrossRef]
- Long, Y.; Yu, G.; Dong, L.; Xu, Y.; Lin, H.; Deng, Y.; You, X.; Yang, L.; Liao, B.-Q. Synergistic fouling behaviors and mechanisms of calcium ions and polyaluminum chloride associated with alginate solution in coagulation-ultrafiltration (UF) process. Water Res. 2021, 189, 116665. [Google Scholar] [CrossRef]
- Pan, Z.; Zeng, B.; Lin, H.; Teng, J.; Zhang, H.; Hong, H.; Zhang, M. Fundamental thermodynamic mechanisms of membrane fouling caused by transparent exopolymer particles (TEP) in water treatment. Sci. Total Environ. 2022, 820, 153252. [Google Scholar] [CrossRef]
- Gao, F.; Peng, Y.-Y.; Li, C.; Cui, W.; Yang, Z.-H.; Zeng, G.-M. Coupled nutrient removal from secondary effluent and algal biomass production in membrane photobioreactor (MPBR): Effect of HRT and long-term operation. Chem. Eng. J. 2018, 335, 169–175. [Google Scholar] [CrossRef]
- Ma, S.; Zeng, W.; Huang, Y.; Zhu, X.; Xia, A.; Zhu, X.; Liao, Q. Revealing the synergistic effects of cells, pigments, and light spectra on light transfer during microalgae growth: A comprehensive light attenuation model. Bioresour. Technol. 2022, 348, 126777. [Google Scholar] [CrossRef] [PubMed]
- Sheng, A.L.K.; Bilad, M.R.; Osman, N.B.; Arahman, N. Sequencing batch membrane photobioreactor for real secondary effluent polishing using native microalgae: Process performance and full-scale projection. J. Clean. Prod. 2017, 168, 708–715. [Google Scholar] [CrossRef]







| Parameters | Value |
|---|---|
| Working volume | 9.64 L |
| Aeration rate | 7.5 ± 0.03 L/min |
| Illumination intensity | 8400 lux |
| SRT | 50 d |
| HRT | 2.9 ± 0.1 d |
| Operating temperature | 25.2 ± 1.0 °C |
| Operating pH | 6.81 ± 0.66 |
| Membrane type | Flat sheet |
| Membrane material | Polyvinylidene fluoride (PVDF) |
| Effective surface area | 0.03 m2 |
| Pore size | 0.1 μm |
| Membrane flux | 7.30 ± 0.34 L/(h·m2) |
| Reagents | Element Concentration (mg/L) |
|---|---|
| Glucose | 500 |
| EDTA disodium salt dehydrate | 64 |
| NH4Cl | 50 (N) |
| K2HPO4 | 3.55 (P) |
| KH2PO4 | 5.9 (P) |
| CaCl2·2H2O | 3.0 (Ca) |
| MnCl2·4H2O | 0.4 (Mn) |
| CoCl2·6H2O | 0.1 (Co) |
| FeSO4·7H2O | 1.0 (Fe) |
| Na2MoO4·2H2O | 0.47 (Mo) |
| ZnSO4·7H2O | 2.0 (Zn) |
| CuSO4·5H2O | 0.4 (Cu) |
| H3BO3 | 2.0 (B) |
| MgSO4·7H2O | 6.0 (Mg) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, H.; Rutta, N.C.; Chen, S.; Zhang, M.; Lin, H.; Liao, B. Membrane Photobioreactor Applied for Municipal Wastewater Treatment at a High Solids Retention Time: Effects of Microalgae Decay on Treatment Performance and Biomass Properties. Membranes 2022, 12, 564. https://doi.org/10.3390/membranes12060564
Zou H, Rutta NC, Chen S, Zhang M, Lin H, Liao B. Membrane Photobioreactor Applied for Municipal Wastewater Treatment at a High Solids Retention Time: Effects of Microalgae Decay on Treatment Performance and Biomass Properties. Membranes. 2022; 12(6):564. https://doi.org/10.3390/membranes12060564
Chicago/Turabian StyleZou, Hui, Neema Christopher Rutta, Shilei Chen, Meijia Zhang, Hongjun Lin, and Baoqiang Liao. 2022. "Membrane Photobioreactor Applied for Municipal Wastewater Treatment at a High Solids Retention Time: Effects of Microalgae Decay on Treatment Performance and Biomass Properties" Membranes 12, no. 6: 564. https://doi.org/10.3390/membranes12060564