Insights into the Role of Biopolymer-Based Xerogels in Biomedical Applications
Abstract
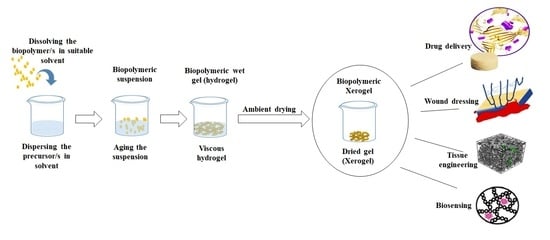
:1. Introduction
2. Xerogel Functional Material
2.1. Fabrication Techniques
2.2. Properties and Advantages of Xerogels
2.3. Suitability of Biopolymeric Xerogels in Biomedical Applications
3. Biopolymeric Xerogels in Biomedical Applications
3.1. Drug Delivery
3.2. Antibacterial and Wound Healing Applications
3.3. Tissue Engineering
3.4. Biosensing
4. Challenges and Future Prospective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yahya, E.B.; Amirul, A.; Abdul Khalil, H.P.S.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; AK, A.S.; Adnan, A. Insights into the role of biopolymer aerogel scaffolds in tissue engineering and regenerative medicine. Polymers 2021, 13, 1612. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; He, X.; Huang, T.; Tang, B.; Cheng, X.; Zhang, Y.; Shao, Z. A facile preparation of transparent methyltriethoxysilane based silica xerogel monoliths at ambient pressure drying. Microporous Mesoporous Mater. 2019, 286, 98–104. [Google Scholar] [CrossRef]
- Darpentigny, C.; Nonglaton, G.; Bras, J.; Jean, B. Highly absorbent cellulose nanofibrils aerogels prepared by supercritical drying. Carbohydr. Polym. 2020, 229, 115560. [Google Scholar] [CrossRef] [PubMed]
- Elma, M.; Setyawan, H. Synthesis of Silica Xerogels Obtained in Organic Catalyst via Sol Gel Route. IOP Conf. Ser. Earth Environ. Sci. 2018, 175, 012008. [Google Scholar] [CrossRef]
- Ptaszkowska-Koniarz, M.; Goscianska, J.; Pietrzak, R. Removal of rhodamine B from water by modified carbon xerogels. Colloids Surf. A Physicochem. Eng. Asp. 2018, 543, 109–117. [Google Scholar] [CrossRef]
- Adamova, L.; Safronov, A.; Terziyan, T.; Shabadrov, P.; Klyukina, A. Thermodynamics of Swelling of Polyacrylamide and Poly (methacrylic acid) Lyophilized Xerogels in Water. Polym. Sci. Ser. A 2018, 60, 190–197. [Google Scholar] [CrossRef]
- Yamasaki, S.; Sakuma, W.; Yasui, H.; Daicho, K.; Saito, T.; Fujisawa, S.; Isogai, A.; Kanamori, K. Nanocellulose Xerogels with high porosities and large specific surface areas. Front. Chem. 2019, 7, 316. [Google Scholar] [CrossRef] [Green Version]
- Kaya, G.G.; Deveci, H. Synergistic effects of silica aerogels/xerogels on properties of polymer composites: A review. J. Ind. Eng. Chem. 2020, 89, 13–27. [Google Scholar] [CrossRef]
- Pramanik, R.; Ganivada, B.; Ram, F.; Shanmuganathan, K.; Arockiarajan, A. Influence of nanocellulose on mechanics and morphology of polyvinyl alcohol xerogels. J. Mech. Behav. Biomed. Mater. 2019, 90, 275–283. [Google Scholar] [CrossRef]
- Awadallah-F, A.; Al-Muhtaseb, S.A. Influence of Chitosan Addition on Resorcinol–Formaldehyde Xerogel Structure. Appl. Sci. 2019, 9, 4582. [Google Scholar] [CrossRef] [Green Version]
- Rbihi, S.; Laallam, L.; Sajieddine, M.; Jouaiti, A. Characterization and thermal conductivity of cellulose based composite xerogels. Heliyon 2019, 5, e01704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groult, S.; Buwalda, S.; Budtova, T. Pectin hydrogels, aerogels, cryogels and xerogels: Influence of drying on structural and release properties. Eur. Polym. J. 2021, 149, 110386. [Google Scholar] [CrossRef]
- Khattab, T.A.; Dacrory, S.; Abou-Yousef, H.; Kamel, S. Development of microporous cellulose-based smart xerogel reversible sensor via freeze drying for naked-eye detection of ammonia gas. Carbohydr. Polym. 2019, 210, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Patil, G.; Torris, A.; Suresha, P.; Jadhav, S.; Badiger, M.V.; Ghormade, V. Design and synthesis of a new topical agent for halting blood loss rapidly: A multimodal chitosan-gelatin xerogel composite loaded with silica nanoparticles and calcium. Colloids Surf. B Biointerfaces 2021, 198, 111454. [Google Scholar] [CrossRef]
- Bilanovic, D.; Starosvetsky, J.; Armon, R.H. Preparation of biodegradable xanthan–glycerol hydrogel, foam, film, aerogel and xerogel at room temperature. Carbohydr. Polym. 2016, 148, 243–250. [Google Scholar] [CrossRef]
- Zhou, H.-J.; Teng, S.-H.; Zhou, Y.-B.; Qian, H.-S. Green Strategy to Develop Novel Drug-Containing Poly (ε-Caprolactone)-Chitosan-Silica Xerogel Hybrid Fibers for Biomedical Applications. J. Nanomater. 2020, 2020, 6659287. [Google Scholar] [CrossRef]
- Kaya, G.G.; Yilmaz, E.; Deveci, H. Synthesis of sustainable silica xerogels/aerogels using inexpensive steel slag and bean pod ash: A comparison study. Adv. Powder Technol. 2020, 31, 926–936. [Google Scholar] [CrossRef]
- Zhu, J.; Xie, J.; Lü, X.; Jiang, D. Synthesis and characterization of superhydrophobic silica and silica/titania aerogels by sol–gel method at ambient pressure. Colloids Surf. A Physicochem. Eng. Asp. 2009, 342, 97–101. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, T.; Xia, X.; Wu, L.; Shao, L.; Zhou, J.; Li, J. How biomimetic amino modified mesoporous silica xerogel regulates loading and in vitro sustained delivery of levorotary ofloxacin. Mater. Sci. Eng. C 2020, 107, 110266. [Google Scholar] [CrossRef]
- Uthappa, U.; Sriram, G.; Brahmkhatri, V.; Kigga, M.; Jung, H.-Y.; Altalhi, T.; Neelgund, G.M.; Kurkuri, M.D. Xerogel modified diatomaceous earth microparticles for controlled drug release studies. New J. Chem. 2018, 42, 11964–11971. [Google Scholar] [CrossRef]
- Antonov, D.O.; Tambasova, D.P.; Shishmakov, A.B.; Kirilyuk, I.A.; Kovaleva, E.G. Acidic and Electrosurface Properties of Binary TiO2-SiO2 Xerogels Using EPR of pH-Sensitive Nitroxides. Gels 2021, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Synthesis, drying process and medical application of polysaccharide-based aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Tao, X.; Qi, Z.; Yin, Z.; Kundu, S.C.; Lu, S. Highly Absorbent Silk Fibroin Protein Xerogel. ACS Biomater. Sci. Eng. 2021, 7, 3594–3607. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef]
- Soorbaghi, F.P.; Isanejad, M.; Salatin, S.; Ghorbani, M.; Jafari, S.; Derakhshankhah, H. Bioaerogels: Synthesis approaches, cellular uptake, and the biomedical applications. Biomed. Pharmacother. 2019, 111, 964–975. [Google Scholar] [CrossRef]
- Salimian, S.; Zadhoush, A.; Talebi, Z. Interpenetrating organic–inorganic network: A short review on aerogel as a nanoporous filler in epoxy nanocomposite. Mater. Des. Process. Commun. 2019, 1, e107. [Google Scholar] [CrossRef] [Green Version]
- Abdul Khalil, H.P.S.; Adnan, A.; Yahya, E.B.; Olaiya, N.; Safrida, S.; Hossain, M.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.; Oyekanmi, A. A review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef]
- Gutierrez Cano, V.; Menelaou, M.; Kastyl, J.; Cihlář, J.; Tkachenko, S.; González, J.A.; Kalmár, J.; Fabian, I.; Lázár, I.; Čelko, L. Rare-Earth Zirconate Ln2Zr2O7 (Ln: La, Nd, Gd, and Dy) Powders, Xerogels, and Aerogels: Preparation, Structure, and Properties. Inorg. Chem. 2019, 58, 14467–14477. [Google Scholar]
- Paladini, G.; Venuti, V.; Crupi, V.; Majolino, D.; Fiorati, A.; Punta, C. FTIR-ATR analysis of the H-bond network of water in branched polyethyleneimine/TEMPO-oxidized cellulose nano-fiber xerogels. Cellulose 2020, 27, 8605–8618. [Google Scholar] [CrossRef]
- Aiello, A.; Cosby, T.; McFarland, J.; Durkin, D.P.; Trulove, P.C. Mesoporous xerogel cellulose composites from biorenewable natural cotton fibers. Carbohydr. Polym. 2022, 282, 119040. [Google Scholar] [CrossRef]
- Toivonen, M.S.; Kaskela, A.; Rojas, O.J.; Kauppinen, E.I.; Ikkala, O. Ambient-dried cellulose nanofibril aerogel membranes with high tensile strength and their use for aerosol collection and templates for transparent, flexible devices. Adv. Funct. Mater. 2015, 25, 6618–6626. [Google Scholar] [CrossRef] [Green Version]
- Melone, L.; Rossi, B.; Pastori, N.; Panzeri, W.; Mele, A.; Punta, C. TEMPO-oxidized cellulose cross-linked with branched polyethyleneimine: Nanostructured adsorbent sponges for water remediation. ChemPlusChem 2015, 80, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Paladini, G.; Venuti, V.; Almásy, L.; Melone, L.; Crupi, V.; Majolino, D.; Pastori, N.; Fiorati, A.; Punta, C. Cross-linked cellulose nano-sponges: A small angle neutron scattering (SANS) study. Cellulose 2019, 26, 9005–9019. [Google Scholar] [CrossRef]
- Yahya, E.B.; Jummaat, F.; Amirul, A.; Adnan, A.; Olaiya, N.; Abdullah, C.; Rizal, S.; Mohamad Haafiz, M.; Khalil, H. A review on revolutionary natural biopolymer-based aerogels for antibacterial delivery. Antibiotics 2020, 9, 648. [Google Scholar] [CrossRef]
- Rizal, S.; Yahya, E.B.; Abdul Khalil, H.P.S.; Abdullah, C.; Marwan, M.; Ikramullah, I.; Muksin, U. Preparation and Characterization of Nanocellulose/Chitosan Aerogel Scaffolds Using Chemical-Free Approach. Gels 2021, 7, 246. [Google Scholar] [CrossRef]
- Niu, N.; Teng, S.-H.; Zhou, H.-J.; Qian, H.-S. Synthesis, characterization, and in vitro drug delivery of chitosan-silica hybrid microspheres for bone tissue engineering. J. Nanomater. 2019, 2019, 7425787. [Google Scholar] [CrossRef] [Green Version]
- Chong, S.; Riley, B.J.; Peterson, J.A.; Olszta, M.J.; Nelson, Z.J. Gaseous iodine sorbents: A comparison between Ag-loaded aerogel and xerogel scaffolds. ACS Appl. Mater. Interfaces 2020, 12, 26127–26136. [Google Scholar] [CrossRef]
- Mariana, M.; Abdul Khalil, H.P.S.; Yahya, E.B.; Olaiya, N.; Alfatah, T.; Suriani, A.; Mohamed, A. Recent trends and future prospects of nanostructured aerogels in water treatment applications. J. Water Process Eng. 2022, 45, 102481. [Google Scholar] [CrossRef]
- Ganesan, K.; Dennstedt, A.; Barowski, A.; Ratke, L. Design of aerogels, cryogels and xerogels of cellulose with hierarchical porous structures. Mater. Des. 2016, 92, 345–355. [Google Scholar] [CrossRef]
- Guzel Kaya, G.; Deveci, H. Effect of aging solvents on physicochemical and thermal properties of silica xerogels derived from steel slag. ChemistrySelect 2020, 5, 1586–1591. [Google Scholar] [CrossRef]
- Prakash, S.S.; Brinker, C.J.; Hurd, A.J.; Rao, S.M. Silica aerogel films prepared at ambient pressure by using surface derivatization to induce reversible drying shrinkage. Nature 1995, 374, 439–443. [Google Scholar] [CrossRef]
- Kanamori, K.; Aizawa, M.; Nakanishi, K.; Hanada, T. New transparent methylsilsesquioxane aerogels and xerogels with improved mechanical properties. Adv. Mater. 2007, 19, 1589–1593. [Google Scholar] [CrossRef]
- Tan, E.; Li, B.L.; Ariga, K.; Lim, C.-T.; Garaj, S.; Leong, D.T. Toxicity of two-dimensional layered materials and their heterostructures. Bioconjug. Chem. 2019, 30, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.O.; Yahya, E.B.; Andleeb, S.; Ahmed, M.M.; Javaid, M.U.; Shakeel, W.; Iqbal, I. In vivo assessment of reversing Cisplatin-Induced nephrotoxicity using Jatropha mollissima crude extract and its potential cytotoxicity. Saudi J. Biol. Sci. 2021, 28, 7373–7378. [Google Scholar] [CrossRef]
- Iskandar, M.; Yahya, E.B.; Abdul Khalil, H.P.S.; Rahman, A.; Ismail, M. Recent Progress in Modification Strategies of Nanocellulose-Based Aerogels for Oil Absorption Application. Polymers 2022, 14, 849. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef]
- Oyekanmi, A.; Abdul Khalil, H.P.S.; Rahman, A.; Mistar, E.; Olaiya, N.; Alfatah, T.; Yahya, E.B.; Mariana, M.; Hazwan, C.; Abdullah, C. Extracted supercritical CO2 cinnamon oil functional properties enhancement in cellulose nanofibre reinforced Euchema cottoni biopolymer films. J. Mater. Res. Technol. 2021, 15, 4293–4308. [Google Scholar] [CrossRef]
- Kenawy, E.; Omer, A.; Tamer, T.; Elmeligy, M.; Eldin, M.M. Fabrication of biodegradable gelatin/chitosan/cinnamaldehyde crosslinked membranes for antibacterial wound dressing applications. Int. J. Biol. Macromol. 2019, 139, 440–448. [Google Scholar] [CrossRef]
- Rizal, S.; Saharudin, N.; Olaiya, N.; ABDUL Khalil, H.P.S.; Haafiz, M.; Ikramullah, I.; Muksin, U.; Olaiya, F.G.; Abdullah, C.; Yahya, E.B. Functional Properties and Molecular Degradation of Schizostachyum Brachycladum Bamboo Cellulose Nanofibre in PLA-Chitosan Bionanocomposites. Molecules 2021, 26, 2008. [Google Scholar] [CrossRef]
- Appuhamillage, G.A.; Berry, D.R.; Benjamin, C.E.; Luzuriaga, M.A.; Reagan, J.C.; Gassensmith, J.J.; Smaldone, R.A. A biopolymer-based 3D printable hydrogel for toxic metal adsorption from water. Polym. Int. 2019, 68, 964–971. [Google Scholar] [CrossRef] [Green Version]
- Yahya, E.B.; Alzalouk, M.M.; Alfallous, K.A.; Abogmaza, A.F. Antibacterial cellulose-based aerogels for wound healing application: A review. Biomed. Res. Ther. 2020, 7, 4032–4040. [Google Scholar] [CrossRef]
- García-González, C.A.; López-Iglesias, C.; Concheiro, A.; Alvarez-Lorenzo, C. Biomedical applications of polysaccharide and protein based aerogels. Biobased Aerogels 2018, 16, 295–323. [Google Scholar]
- Heinemann, S.; Heinemann, C.; Bernhardt, R.; Reinstorf, A.; Nies, B.; Meyer, M.; Worch, H.; Hanke, T. Bioactive silica–collagen composite xerogels modified by calcium phosphate phases with adjustable mechanical properties for bone replacement. Acta Biomater. 2009, 5, 1979–1990. [Google Scholar] [CrossRef] [PubMed]
- Bailon-Garcia, E.; Maldonado-Hodar, F.J.; Carrasco-Marin, F.; Perez-Cadenas, A.F.; Bosi, S.; Prato, M. The use of functionalized carbon xerogels in cells growth. Mater. Sci. Eng. C 2019, 100, 598–607. [Google Scholar] [CrossRef]
- Dai, C.; Liu, C.; Wei, J.; Hong, H.; Zhao, Q. Molecular imprinted macroporous chitosan coated mesoporous silica xerogels for hemorrhage control. Biomaterials 2010, 31, 7620–7630. [Google Scholar] [CrossRef]
- Elshishiny, F.; Mamdouh, W. Fabrication of Nanofibrous/Xerogel Layer-by-Layer Biocomposite Scaffolds for Skin Tissue Regeneration: In Vitro Study. ACS Omega 2020, 5, 2133–2147. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; García-González, C.A. 3D-printed alginate-hydroxyapatite aerogel scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2021, 131, 112525. [Google Scholar] [CrossRef]
- Lee, E.J.; Jun, S.H.; Kim, H.E.; Koh, Y.H. Collagen–silica xerogel nanohybrid membrane for guided bone regeneration. J. Biomed. Mater. Res. A 2012, 100, 841–847. [Google Scholar] [CrossRef]
- Jummaat, F.; Yahya, E.B.; Abdul Khalil, H.P.S.; Adnan, A.S.; Alqadhi, A.M.; Abdullah, C.K.; A.K., A.S.; Olaiya, N.; Abdat, M. The role of biopolymer-based materials in obstetrics and gynecology applications: A review. Polymers 2021, 13, 633. [Google Scholar] [CrossRef]
- Zhao, Q.; Lin, Y.; Han, N.; Li, X.; Geng, H.; Wang, X.; Cui, Y.; Wang, S. Mesoporous carbon nanomaterials in drug delivery and biomedical application. Drug Deliv. 2017, 24, 94–107. [Google Scholar] [CrossRef]
- Rajalekshmy, G.; Rekha, M. Synthesis and evaluation of an alginate-methacrylate xerogel for insulin delivery towards wound healing applications. Ther. Deliv. 2021, 12, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Rafati, A.; Ebadi, A.; Bavafa, S.; Nowroozi, A. Kinetic study, structural analysis and computational investigation of novel xerogel based on drug-PEG/SiO2 for controlled release of enrofloxacin. J. Mol. Liq. 2018, 266, 733–742. [Google Scholar] [CrossRef]
- Križman, K.; Novak, S.; Kristl, J.; Majdič, G.; Drnovšek, N. Long-acting silk fibroin xerogel delivery systems for controlled release of estradiol. J. Drug Deliv. Sci. Technol. 2021, 65, 102701. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; d’Ayala, G.G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [Green Version]
- Dai, T.; Wang, C.; Wang, Y.; Xu, W.; Hu, J.; Cheng, Y. A nanocomposite hydrogel with potent and broad-spectrum antibacterial activity. ACS Appl. Mater. Interfaces 2018, 10, 15163–15173. [Google Scholar] [CrossRef]
- Yahya, E.; Abdulsamad, M.A. In-vitro Antibacterial Activity of Carbopol-Essential Oils hydrogels. J. Appl. Sci. Process Eng. 2020, 7, 564–571. [Google Scholar] [CrossRef]
- Rajalekshmy, G.; Rekha, M. Strontium ion cross-linked alginate-g-poly (PEGMA) xerogels for wound healing applications: In vitro studies. Carbohydr. Polym. 2021, 251, 117119. [Google Scholar]
- Correa-Gallegos, D.; Jiang, D.; Christ, S.; Ramesh, P.; Ye, H.; Wannemacher, J.; Kalgudde Gopal, S.; Yu, Q.; Aichler, M.; Walch, A. Patch repair of deep wounds by mobilized fascia. Nature 2019, 576, 287–292. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, C.-Y.; Qu, D.-H.; Long, Y.-T.; Feringa, B.L.; Tian, H. Exploring a naturally tailored small molecule for stretchable, self-healing, and adhesive supramolecular polymers. Sci. Adv. 2018, 4, eaat8192. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Liu, X.; Li, Z.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Zhu, S.; Wu, S. Rapid bacteria capturing and killing by AgNPs/N-CD@ ZnO hybrids strengthened photo-responsive xerogel for rapid healing of bacteria-infected wounds. Chem. Eng. J. 2021, 414, 128805. [Google Scholar] [CrossRef]
- Banu, A.; Gousuddin, M.; Yahya, E.B. Green synthesized monodispersed silver nanoparticles’ characterization and their efficacy against cancer cells. Biomed. Res. Ther. 2021, 8, 4476–4482. [Google Scholar] [CrossRef]
- Deon, M.; Morawski, F.; Passaia, C.; Dalmás, M.; Laranja, D.; Malheiros, P.; Nicolodi, S.; Arenas, L.; Costa, T.; de Menezes, E. Chitosan-stabilized gold nanoparticles supported on silica/titania magnetic xerogel applied as antibacterial system. J. Sol-Gel Sci. Technol. 2019, 89, 333–342. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, H.; Tuo, X.; Guo, H.; Xu, P.; Liu, D.; Wei, Y.; Liu, H.; Fan, Y.; Guo, X. A porous sodium polyacrylate-grafted chitosan xerogel for severe hemorrhage control synthesized from one-pot reaction. J. Mater. Chem. B 2017, 5, 4845–4851. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Lu, B.; Wang, T.; Wang, L.; Chen, J.; Yu, K.; Liu, J.; Dai, F.; Wu, D. Chitosan/gelatin composite sponge is an absorbable surgical hemostatic agent. Colloids Surf. B Biointerfaces 2015, 136, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, Y.; SU, X. Preparation of porous biodegradable chitosan/berberine hydrochloride composite xerogel and its antibacterial and hemostatic properties. Chin. J. Tissue Eng. Res. 2017, 53, 899–905. [Google Scholar]
- Mirtaghavi, A.; Luo, J.; Muthuraj, R. Recent Advances in Porous 3D Cellulose Aerogels for Tissue Engineering Applications: A Review. Journal of Composites Science 2020, 4, 152. [Google Scholar] [CrossRef]
- Wu, X.; Yan, F.; Liu, W.; Zhan, H.; Yang, W. Synthesis and characterization of silk fibroin-bioactive glass hybrid xerogels. Biomater. Biomech. Bioeng. 2014, 1, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Rößler, S.; Brückner, A.; Kruppke, I.; Wiesmann, H.-P.; Hanke, T.; Kruppke, B. 3D Plotting of Silica/Collagen Xerogel Granules in an Alginate Matrix for Tissue-Engineered Bone Implants. Materials 2021, 14, 830. [Google Scholar] [CrossRef]
- Khattab, T.A.; Fouda, M.M.; Rehan, M.; Okla, M.K.; Alamri, S.A.; Alaraidh, I.A.; Al-Ghamdi, A.A.; Soufan, W.H.; Abdelsalam, E.M.; Allam, A.A. Novel halochromic cellulose nanowhiskers from rice straw: Visual detection of urea. Carbohydr. Polym. 2020, 231, 115740. [Google Scholar] [CrossRef]
- Pottathara, Y.B.; Bobnar, V.; Finšgar, M.; Grohens, Y.; Thomas, S.; Kokol, V. Cellulose nanofibrils-reduced graphene oxide xerogels and cryogels for dielectric and electrochemical storage applications. Polymer 2018, 147, 260–270. [Google Scholar] [CrossRef]
- Holthoff, E.L.; Bright, F.V. Molecularly imprinted xerogels as platforms for sensing. Acc. Chem. Res. 2007, 40, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, J.C.; Faustini, M.; Garrido, J.J. Effects of the porous texture and surface chemistry of silica xerogels on the sensitivity of fiber-optic sensors toward VOCs. Sens. Actuators B Chem. 2016, 222, 1166–1174. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, F.; Liang, X.; Dai, G.; Qu, F. Abundant defects of zirconium-organic xerogels: High anhydrous proton conductivities over a wide temperature range and formic acid impedance sensing. J. Colloid Interface Sci. 2022, 607, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-T.; Wu, C.-L. Electrical sensing properties of silica aerogel thin films to humidity. Thin Solid Film. 2006, 496, 658–664. [Google Scholar] [CrossRef]
- Freeman, M.H.; Hall, J.R.; Leopold, M.C. Monolayer-protected nanoparticle doped xerogels as functional components of amperometric glucose biosensors. Anal. Chem. 2013, 85, 4057–4065. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, S.; El-Naggar, M.E.; Abu-Saied, M.; Khattab, T.A.; Saleh, D.I. Preparation of biosensor based on triarylmethane loaded cellulose acetate xerogel for the detection of urea. Mater. Chem. Phys. 2022, 276, 125377. [Google Scholar] [CrossRef]
- Abdelrahman, M.S.; Khattab, T.A.; Aldalbahi, A.; Hatshan, M.R.; El-Naggar, M.E. Facile development of microporous cellulose acetate xerogel immobilized with hydrazone probe for real time vapochromic detection of toxic ammonia. J. Environ. Chem. Eng. 2020, 8, 104573. [Google Scholar] [CrossRef]
- Zakerzadeh, E.; Alizadeh, E.; Samadi Kafil, H.; Mohammad Hassanzadeh, A.; Salehi, R.; Mahkam, M. Novel antibacterial polymeric nanocomposite for smart co-delivery of anticancer drugs. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1509–1520. [Google Scholar] [CrossRef] [Green Version]
- Riss, T.; Niles, A.; Moravec, R.; Karassina, N.; Vidugiriene, J. Cytotoxicity assays: In vitro methods to measure dead cells. In Assay Guidance Manual; Eli Lilly & Company and the National Center for Advancing Translational: Bethesda, MD, USA, 2019. [Google Scholar]






| Type of Xerogel | Experiment | Type of Cells | Conclusion | Ref |
|---|---|---|---|---|
| Chitosan-gelatin xerogel | Hemocompatibility, cytotoxicity assays | Mouse embryonic fibroblast cells | Good platelet activation, good biocompatibility, and thrombin generation activities. | [14] |
| Collagen-silica xerogel | Cell culture experiments | Human monocytes | The xerogel promoted the differentiation of monocytes into osteoclast-like cells. | [53] |
| Carbon xerogel | Cytotoxicity test | Fibroblast cell | The xerogel was biocompatible; the presence of carbon fibers increases the cell’s proliferation. | [54] |
| Chitosan coated mesoporous silica xerogels | Cytotoxicity assays | Mouse myoblast cells line | No obvious cytotoxicity was reported for the xerogel even after 7 days of the exposure. | [55] |
| Silk Fibroin Protein Xerogel | Hemostasis experiments | In-vitro and in-vivo rabbit ear | Good hemostatic properties were observed both in vitro and in vivo for the xerogel. | [23] |
| Chitosan–poly(vinyl alcohol) xerogel | Cytotoxicity and migration rate | Mouse embryonic fibroblast | The xerogel exhibited significant cell proliferation & migration rates and high biocompatibility. | [56] |
| Alginate-hydroxyapatite aerogel | Cytotoxicity, viability, and migration | Mesenchymal stem cells | Highly biocompatible, allowed attachment and migration. | [57] |
| Collagen–silica xerogel | Cell proliferation assay | Preosteoblast cells | Good biocompatibility and high level of osteoblast differentiation | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Khalil, H.P.S.; Yahya, E.B.; Tajarudin, H.A.; Balakrishnan, V.; Nasution, H. Insights into the Role of Biopolymer-Based Xerogels in Biomedical Applications. Gels 2022, 8, 334. https://doi.org/10.3390/gels8060334
Abdul Khalil HPS, Yahya EB, Tajarudin HA, Balakrishnan V, Nasution H. Insights into the Role of Biopolymer-Based Xerogels in Biomedical Applications. Gels. 2022; 8(6):334. https://doi.org/10.3390/gels8060334
Chicago/Turabian StyleAbdul Khalil, H. P. S., Esam Bashir Yahya, Husnul Azan Tajarudin, Venugopal Balakrishnan, and Halimatuddahliana Nasution. 2022. "Insights into the Role of Biopolymer-Based Xerogels in Biomedical Applications" Gels 8, no. 6: 334. https://doi.org/10.3390/gels8060334