Coordination Polymers in Dicyanamido-Cadmium(II) with Diverse Network Dimensionalities
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Physical Measurements
2.2. Preparation of the Compounds
2.3. Single-Crystal Structure Determination
3. Results and Discussion
3.1. Synthetic Aspects and IR Spectra of the Complexes
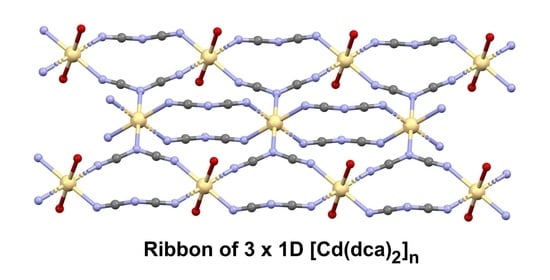
3.2. Description of the Structures
3.2.1. Catena-[Cd(μ1,3-dca) (μ1,5-dca)(3-ampy)] (1)
3.2.2. Catena-[Cd3(μ1,3,5-dca)2(μ1,5-dca)4(pyNO)2(H2O)2] (2)
3.2.3. Catena-{[Cd(H2O)2(μ1,5-dca)2](2,6-lut-NO)} (3)
3.2.4. Catena-[Cd(Me2en)(μ1,5-dca)2] (4)
3.2.5. Catena-[Cd(Me4en)(μ1,5-dca)2] (5)
3.2.6. [Cd(1,8-damnph)2(dca)2] (6)
3.3. Luminescence Emission
3.4. Thermal Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sigel, A.; Sigel, H.; Sigel, R.K.O. (Eds.) Metal Ions in Life Sciences: Cadmium: From Toxicity to Essentiality: (a) Complex formation of cadmium with sugar residues, nucleobases, phosphates, nucleotides, and nucleic Acids by Sigel, R.K.O.; (b) Cadmium(II) complexes of amino acids and peptides by Sóvágó, I.; Várnagy, K.; Book Series (MILS); Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2011; Volume 11, p. 275. [Google Scholar]
- Massoud, S.S.; Louka, F.R.; Obaid, Y.K.; Vicente, R.; Ribas, J.; Fischer, R.C.; Mautner, F.A. Metal ions directing the geometry and nuclearity of azido-metal(II) complexes derived from bis(2-(3,5-dimethyl-1H-pyrazol-1-yl)ethyl)amine. Dalton Trans. 2013, 42, 3968–3978. [Google Scholar] [CrossRef] [PubMed]
- Mautner, F.A.; Louka, F.R.; Hofer, J.; Spell, M.; Lefèvre, A.; Guilbeau, A.E.; Massoud, S.S. One-dimensional cadmium polymers with alternative di(EO/EE) and di(EO/EO/EO/EE) bridged azide bonding modes. Cryst. Growth Des. 2013, 13, 4518–4525. [Google Scholar] [CrossRef]
- Mautner, F.A.; Scherzer, M.; Berger, C.; Fischer, R.C.; Vicente, R.; Massoud, S.S. Synthesis and characterization of three new 1-D polymeric [M2(4-azidopyridine)4(µ1,1-N3)2(µ1,3-N3)2]n (M = Ni, Co, Cd) complexes. Polyhedron 2015, 85, 329–336. [Google Scholar] [CrossRef]
- Mautner, F.A.; Berger, C.; Fischer, R.C.; Massoud, S.S. Synthesis, characterization and luminescence properties of zinc(II) and cadmium(II) pseudohalide complexes derived from quinoline-N-oxide. Inorg. Chim. Acta 2016, 439, 69–76. [Google Scholar] [CrossRef]
- Mautner, F.A.; Berger, C.; Fischer, R.C.; Massoud, S.S. Coordination polymers of azido and thiocyanato Cd(II) and Zn(II) complexes based on 2,6-lutidine-N-oxide. Synthesis, characterization and luminescent properties. Inorg. Chim. Acta 2016, 448, 34–41. [Google Scholar] [CrossRef]
- Mautner, F.A.; Fischer, R.C.; Reichmann, K.; Gullett, E.; Ashkar, K.; Massoud, S.S. Synthesis and characterization of 1D and 2D cadmium(II)-2,2’-bipyridine-N,N’-dioxide coordination polymers bridged by pseudohalides. J. Mol. Struct. 2019, 1175, 797–803. [Google Scholar] [CrossRef]
- Majumdar, D.; Philip, J.E.; Das, S.; Kundu, B.K.; Saini, R.V.; Chandan, G.; Kalipad, B.; Mishr, D. Experimental and theoretical corroboration of antimicrobial and anticancer activities of two pseudohalides induced structurally diverse Cd(II)-Salen complexes. J. Mol. Struct. 2021, 1225, 129189. [Google Scholar] [CrossRef]
- Schulz, A.; Villinger, A. Binary polyazides of cadmium and mercury. Chem. A Eur. J. 2015, 21, 3649. [Google Scholar] [CrossRef] [PubMed]
- Escuer, A.; Esteban, J.; Perlepes, S.P.; Stamatatos, T.C. The bridging azido ligand as a central “player” in high-nuclearity 3d-metal cluster chemistry. Coord. Chem. Rev. 2014, 275, 87–129. [Google Scholar] [CrossRef]
- Jochim, A.; Gallo, G.; Dinnebier, R.; Näther, C. Synthesis, crystal structure and properties of Cd(NCS)2 coordination compounds with two different Cd coordination modes. Z. Naturforsch. 2019, 74, 49–58. [Google Scholar] [CrossRef]
- Neumann, T.; Gallo, G.; Jess, I.; Dinnebier, R.E.; Näther, C. Thermodynamically stable and metastable coordination polymers synthesized from solution and the solid state. Cryst EngComm 2020, 22, 184–194. [Google Scholar] [CrossRef] [Green Version]
- Mautner, F.A.; Scherzer, M.; Berger, C.; Fischer, R.C.; Vicente, R.; Massoud, S.S. Synthesis and characterization of five new thiocyanato- and cyanato-metal(II) complexes with 4-azidopyridine as co-ligand. Polyhedron 2015, 85, 20–26. [Google Scholar] [CrossRef]
- Mautner, F.A.; Jantscher, P.; Fischer, R.C.; Torvisco, A.; Vicente, R.; Karsili, T.N.V.; Massoud, S.S. Synthesis and characterization of 1D coordination polymers of metal(II)-dicyanamido complexes. Polyhedron 2019, 166, 36–43. [Google Scholar] [CrossRef]
- Cui, P.; Chen, Z.; Gao, D.; Zhao, B.; Shi, W.; Cheng, P. Syntheses, structures, and photoluminescence of a series of three-dimensional Cd(II) frameworks with a flexible ligand, 1,5-bis(5-tetrazolo)-3-oxapentane. Cryst. Growth Des. 2010, 10, 4370–4378. [Google Scholar] [CrossRef]
- Mautner, F.A.; Fischer, R.C.; Williams, B.R.; Massoud, S.S.; Salem, N.M.H. Hexnuclear cadmium(ii) cluster constructed from tris(2-methylpyridyl)amine (TPA) and azides. Crystals 2020, 10, 317. [Google Scholar] [CrossRef] [Green Version]
- Aguirre-Díaz, F.A.; Reinares-Fisac, D.; Iglesias, M.; Gutiérrez-Puebla, E.; Gándara, F.; Snejko, N.; Monge, M.Á. Group 13th metal-organic frameworks and their role in heterogeneous catalysis. Coord. Chem. Rev. 2017, 335, 1–27. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Lin, W. Metal–organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane storage in metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef]
- Qiu, S.; Xue, M.; Zhu, G. Metal–organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, B.N.; Bhar, K.; Chattopadhyay, S.; Das, S.; Mitra, P.; Ghosh, B.K. Synthesis, structure and luminescence behaviour of heptacoordinated one-dimensional coordination polymers of the type [Cd(L)(dca)]n(X)n (L = a pentadentate Schiff base; dca = dicyanamide; X =, ). J. Mol. Struct. 2010, 963, 35–40. [Google Scholar] [CrossRef]
- Chattopadhyay, T.; Mahavidyalaya, P.; Banerjee, A.; Banu, K.S.; Eringathodi, S. Cadmium–halide and mixed cadmium–halide–dicyanamide polymers mediated by ancillary 2-aminoalkyl-pyridine ligands: Synthesis, X-ray single crystal structures and luminescence property. Polyhedron 2008, 27, 2452–2458. [Google Scholar] [CrossRef]
- Mautner, F.A.; Traber, M.; Fischer, R.C.; Massoud, S.S.; Vicente, R. Synthesis, crystal structures, spectral and magnetic properties of 1-D polymeric dicyanamido metal(II) complexes. Polyhedron 2017, 138, 13–20. [Google Scholar] [CrossRef]
- Das, D.; Bhar, K.; Fun, H.-K.; Chantrapromma, S.; Ghosh, B.K. Syntheses, characterizations and structures of complexes of the types mononuclear [MII(tren)(dca)]ClO4 [M = Cu and Zn; tren = tris(2-aminoethyl)amine; dca = dicyanamide] and dinuclear [CdII2(tren)2(dca)](ClO4)3: Variation of nuclearities and architectures with metal-ion templates. Inorg. Chim. Acta 2010, 363, 784–792. [Google Scholar]
- Ding, B.; Li, J.; Yang, E.-C.; Wang, X.-G.; Zhao, X.-J. Synthesis, structure and characterization of a novel one-dimensional tube-like cadmium coordination polymer. Z. Anorg. Allg. Chem. 2007, 633, 1062–1065. [Google Scholar] [CrossRef]
- Jurgens, B.; Irran, E.; Hoppe, H.A.; Schnick, W. Phase transition of a dicyanamide with rutile-like structure: Syntheses and crystal structures of α- and β-Cd[N(CN)2]2. Z. Anorg. Allg. Chem. 2004, 630, 219–223. [Google Scholar] [CrossRef]
- Batten, R.S.; Murray, K.S. Structure and magnetism of coordination polymers containing dicyanamide and tricyanomethanide. Coord. Chem. Rev. 2003, 246, 103–130. [Google Scholar] [CrossRef]
- Mautner, F.A.; Jantscher, P.; Fischer, R.C.; Torvisco, A.; Vicente, R.; Karsili, T.N.V.; Massoud, S.S. Structure, DFT calculations and magnetic characterization of coordination polymers of bridged dicyanamido-metal(II) complexes. Magnetochemistry 2019, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.-Z.; Liang, X.-X.; Cai, Y.; Wu, J.; Shi, Z.-F.; Kirillov, A.M. Hydrothermal assembly, structures, topologies, luminescence, and magnetism of a novel series of coordination polymers driven by a trifunctional nicotinic acid building block. Dalton Trans. 2017, 46, 10908–10925. [Google Scholar] [CrossRef]
- Heine, J.; Müller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal–organic frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Luo, Z.-D.; Pan, Y.; Singh, A.K.; di Trive, M.; Kumar, A. Recent developments in luminescent coordination polymers: Designing strategies, sensing application and theoretical evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Matveevskaya, V.; Pavlov, D.; Yakunenkov, A.; Potapov, A. Coordination polymers based on highly emissive ligands: Synthesis and functional properties. Materials 2020, 13, 2699. [Google Scholar] [CrossRef]
- Yi, X.-C.; Huang, M.-X.; Qi, Y.; Gao, E.-Q. Synthesis, structure, luminescence and catalytic properties of cadmium(ii) coordination polymers with 9H-carbazole-2,7-dicarboxylic acid. Dalton Trans. 2014, 43, 3691–3697. [Google Scholar] [CrossRef]
- Xue, L.-P.; Li, Z.-H.; Ma, L.-F.; Wang, L.-Y. Crystal engineering of cadmium coordination polymers decorated with nitro-functionalized thiophene-2,5-dicarboxylate and structurally related bis(imidazole) ligands with varying flexibility. CrystEngComm 2015, 17, 6441–6449. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumar, S.K.; Anantharaman, G. Crystal engineering of zinc and cadmium coordination polymers via a mixed-ligand strategy regulated by aromatic rigid/flexible dicarboxylate ancillary ligands and metal ionic radii: Tuning structure, dimension and photoluminescence properties. Polyhedron 2016, 119, 55–70. [Google Scholar] [CrossRef]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis, and Applications; RSC: Cambridge, UK, 2009. [Google Scholar]
- MacGillery, C.M.; Lukehart, C.M. (Eds.) Metal-Organic Framework Materials; John Wiley & Sons: Chichester, UK, 2014. [Google Scholar]
- Bruker APEX, SAINT v. 8.37A; Bruker AXS Inc.: Madison, WI, USA, 2015.
- Sheldrick, G.M. SADABS v. 2; University of Goettingen: Goettingen, Germany, 2001. [Google Scholar]
- Sheldrick, G.M. A Short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, T.; van de Streek, J.J. Mercury: Visualization and analysis of crystal structures. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. PLATON, a Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 1999. [Google Scholar]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586, (v. 5.4.0.2, release date: 21.04.2020). [Google Scholar] [CrossRef]
- Köhler, H.; Kolbe, A.; Lux, G. Metall-pseudohalogenide. 27. Zur Struktur der Dicyanamide zweiwertiger 3d-metalle M(N(CN)2)2. Z. Anorg. Allg. Chem. 1977, 428, 103–112. [Google Scholar] [CrossRef]
- Chowdhury, H.; Bieńko, A.; Rizzoli, C.; Adhikary, C. Syntheses, structures and magnetic behaviors of 1D and 3D µ1,5-dicyanamide bridged copper(II) coordination polymers containing a symmetrical 1,2-diamine as a chelator. Polyhedron 2020, 188, 114693. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley: New York, NY, USA, 1986; and Part B, 5th ed.; 1997; 2nd ed, 1978. [Google Scholar]
- Jensen, P.; Batten, S.R.; Fallon, G.D.; Moubaraki, B.; Murray, K.S.; Price, D.J. Structural isomers of M(dca)2 molecule-based magnets. Crystal structure of tetrahedrally coordinated sheet-like β-Zn(dca)2 and β-Co/Zn(dca)2, and the octahedrally coordinated rutile-like a-Co(dca)2, where dca– = dicyanamide, N(CN)2–, and magnetism of b-Co(dca)2. Chem. Commun. 1999, 177–178. [Google Scholar] [CrossRef]
- Ogawa, H.; Mori, K.; Murashima, K.; Karasawa, S.; Koga, N. One-, two-, and three-dimensional heterospin complexes consisting of 4-(N-tert-butyloxylamino)pyridine (4NOpy), dicyanamide ion (DCA), and 3d metal Ions: Crystal structures and magnetic properties of [MII(4NOpy)x(DCA)y(CH3CN)z]n (M = Mn, Co, Ni, Cu, Zn). Inorg. Chem. 2016, 55, 717–728. [Google Scholar] [CrossRef]
- Wang, R.; Han, L.; Xu, L.; Gong, Y.; Zhou, Y.; Hong, M.; Chan, A.S.C. Syntheses and characterizations of metal-organic frameworks with unusual topologies derived from flexible dipyridyl ligands. Eur. J. Inorg. Chem. 2004, 2004, 3751–3763. [Google Scholar] [CrossRef]
- Mautner, F.A.; Jantscher, P.V.; Fischer, R.C.; Torvisco, A.; Reichmann, K.; Massoud, S.S. Syntheses, Structural Characterization, and Thermal Behaviour of Metal Complexes with 3-Aminopyridine as Co-ligands. Transition Met. Chem. 2020. [Google Scholar] [CrossRef]
- Chakraborty, P.; Mondal, S.; Das, S.; Jana, A.D.; Das, D. Dicyanamide mediated construction of 1D polymeric networks of quinoxaline with d10 metal ions: Synthesis, thermogravimetric analysis, photoluminescence and a theoretical investigation on the π⋯π interactions. Polyhedron 2014, 70, 11–19. [Google Scholar] [CrossRef]








| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C9H6CdN8 | C22H14Cd3N20O4 | C11H13CdN7O3 |
| Formula mass | 338.62 | 959.76 | 403.69 |
| System | Monoclinic | Triclinic | Triclinic |
| Space group | Cc | P-1 | P-1 |
| a (Å) | 14.3557(7) | 6.6636(12) | 7.4407(4) |
| b (Å) | 11.0239(6) | 7.7098(17) | 7.6936(4) |
| c (Å) | 7.3916(4) | 16.035(3) | 15.6387(8) |
| α (°) | 90 | 77.982(9) | 88.291(2) |
| β (°) | 104.588(2) | 78.672(9) | 86.251(2) |
| γ (°) | 90 | 86.459(12) | 61.252(2) |
| V (Å3) | 1132.05(10) | 789.9(3) | 783.22(7) |
| Z | 4 | 1 | 2 |
| Dcalc (Mg/m3) | 1.987 | 2.018 | 1.712 |
| θ max (°) | 26.992 | 30.309 | 30.040 |
| Data collected | 25470 | 15429 | 22948 |
| Unique refl./Rint | 2441/0.0657 | 4675/0.0441 | 4564/0.0455 |
| Parameters/Restraints | 169/4 | 231/0 | 212/10 |
| Goodness-of-Fit on F2 | 1.036 | 1.162 | 1.333 |
| R1/wR2 (all data) | 0.0176/0.0414 | 0.0261/0.0606 | 0.0388/0.0921 |
| Residual extrema (e/Å3) | 0.517/−0.603 | 0.936/−1.352 | 0.744/−1.168 |
| Compound | 4 | 5 | 6 |
| Empirical formula | C8H12CdN8 | C10H16CdN8 | C24H20CdN10 |
| Formula mass | 332.67 | 360.72 | 560.90 |
| System | Monoclinic | Orthorhombic | Monoclinic |
| Space group | P21/c | Pnma | P21/n |
| a (Å) | 7.4137(3) | 16.8193(7) | 7.6234(3) |
| b (Å) | 13.8858(5) | 11.9291(6) | 11.1927(4) |
| c (Å) | 12.2685(5) | 7.6217(3) | 13.3631(5) |
| α (°) | 90 | 90 | 90 |
| β (°) | 90.251(2) | 90 | 99.803(2) |
| γ (°) | 90 | 90 | 90 |
| V (Å3) | 1262.97(9) | 1529.21(12) | 1125.90(7) |
| Z | 4 | 4 | 2 |
| Dcalc (Mg/m3) | 1.750 | 1.567 | 1.656 |
| θ max (°) | 33.274 | 30.549 | 33.303 |
| Data collected | 107447 | 97158 | 106749 |
| Unique refl./Rint | 4849/0.0852 | 2444/0.0509 | 4323/0.0642 |
| Parameters/Restraints | 164/0 | 130/0 | 176/0 |
| Goodness-of-Fit on F2 | 1.042 | 1.108 | 1.050 |
| R1/wR2 (all data) | 0.0190/0.0491 | 0.0244/0.0618 | 0.0192/0.0517 |
| Residual extrema (e/Å3) | 0.792/−0.934 | 1.084/−0.657 | 0.800/−0.694 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mautner, F.A.; Jantscher, P.V.; Fischer, R.C.; Torvisco, A.; Reichmann, K.; Salem, N.M.H.; Gordon, K.J.; Louka, F.R.; Massoud, S.S. Coordination Polymers in Dicyanamido-Cadmium(II) with Diverse Network Dimensionalities. Crystals 2021, 11, 181. https://doi.org/10.3390/cryst11020181
Mautner FA, Jantscher PV, Fischer RC, Torvisco A, Reichmann K, Salem NMH, Gordon KJ, Louka FR, Massoud SS. Coordination Polymers in Dicyanamido-Cadmium(II) with Diverse Network Dimensionalities. Crystals. 2021; 11(2):181. https://doi.org/10.3390/cryst11020181
Chicago/Turabian StyleMautner, Franz A., Patricia V. Jantscher, Roland C. Fischer, Ana Torvisco, Klaus Reichmann, Nahed M. H. Salem, Kenneth J. Gordon, Febee R. Louka, and Salah S. Massoud. 2021. "Coordination Polymers in Dicyanamido-Cadmium(II) with Diverse Network Dimensionalities" Crystals 11, no. 2: 181. https://doi.org/10.3390/cryst11020181