Unveiling the Antioxidant Therapeutic Functionality of Sustainable Olive Pomace Active Ingredients
Abstract
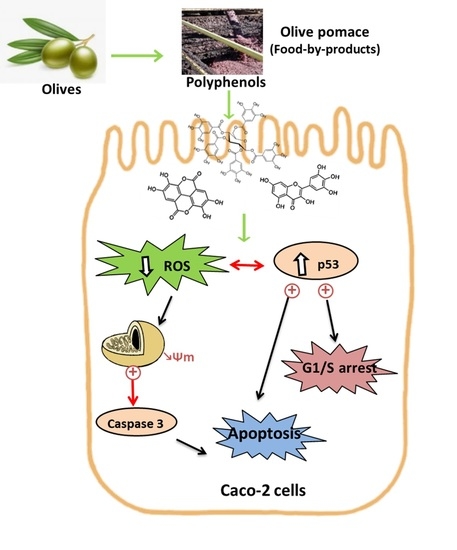
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Determination of the OP Waste Proximate Composition
2.3. Extraction of Bioactive Compounds from Olive Pomace Wastes
2.3.1. Ohmic Heating (OH)
2.3.2. Conventional Heating (CH)
2.4. Analytical Methodology for Extracts Characterization
2.4.1. Extraction Yield
2.4.2. Total Phenolic Compounds
2.4.3. Individual Phenolic Compounds Determination
2.4.4. Antioxidant Activity
2.4.5. Structural Characterization
2.5. Theoretical Absorption Percentage of Individual Phenolic Compounds
2.6. Cell Culture
2.7. Cell Treatment and Antiproliferative Property Analysis
2.8. Measurements of Apoptosis
2.9. Propidium Iodide Stainning of DNA Content and Cell Cycle Analysis
2.10. Flow Cytometry Mitochondrial Membrane Potential Assay
2.11. Determination of Caspase 3 and P53
2.12. Determination of Intracellular Levels of Reactive Oxygen Species (ROS)
2.13. Statistical Analysis
3. Results and Discussion
3.1. Chemical and Mineral Composition of OP
3.2. Extraction Yield, Phenolic Composition, and Cell-Free Antioxidant Activity of OP Extracts
3.3. Structural Characterization of OP Material and OP Extracts
3.4. Theoretical Absorption Percentage of Individual Phenolic Compounds (Based on Lipinski Parameters)
3.5. Effect of OP Extracts on Cancer Cells
3.5.1. Cell Viability Studies
3.5.2. Cell Death Studies
3.5.3. Involvement of ROS in OP Caco-2 Effect
3.6. Antioxidant Capacity of Olive Pomace Extracts on a Model Intestinal Barrier
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Nunzio, M.; Picone, G.; Pasini, F.; Caboni, M.F.; Gianotti, A.; Bordoni, A.; Capozzi, F. Olive oil industry by-products. Effects of a polyphenol-rich extract on the metabolome and response to inflammation in cultured intestinal cell. Food Res. Int. 2018, 113, 392–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, M.; Sathiavelu, S. Waste management in the olive oil industry in the Mediterranean region by composting. Clean Technol. Environ. Policy 2009, 11, 293–298. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Souilem, S.; El-Abbassi, A.; Kiai, H.; Hafidi, A.; Sayadi, S.; Galanakis, C.M. Olive oil production sector: Environmental effects and sustainability challenges. In Olive Mill Waste: Recent Advances for Sustainable Management; Galanakis, C.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 1–28. ISBN 9780128053140. [Google Scholar]
- Galanakis, C.M.; Yücetepe, A.; Kasapoğlu, K.; Özçelik, B. High-value compounds from olive oil processing waste. In Olis Extraction, Processing and Applications; Chemat, S., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 179–203. [Google Scholar]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Mallamaci, R.; Budriesi, R.; Clodoveo, M.L.; Biotti, G.; Micucci, M.; Ragusa, A.; Curci, F.; Muraglia, M.; Corbo, F.; Franchini, C. Olive tree in circular economy as a source of secondary metabolites active for human and animal health beyond oxidative stress and inflammation. Molecules 2021, 26, 1072. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Nieto, G.; Pateiro, M.; Lorenzo, J.M. Phenolic compounds obtained from olea europaea by-products and their use to improve the quality and shelf life of meat and meat products—A review. Antioxidants 2020, 9, 1061. [Google Scholar] [CrossRef]
- Martínez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Osada, J. Could squalene be an added value to use olive by-products? J. Sci. Food Agric. 2020, 100, 915–925. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H. Bin Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Ieri, F.; Bernini, R. Sustainability, innovation, and green chemistry in the production and valorization of phenolic extracts from Olea europaea L. Sustainability 2016, 8, 1002. [Google Scholar] [CrossRef] [Green Version]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Xu, X.; Lu, Y.; Jin, H.; Yang, R.; Jiang, C.; Shao, D.; Liu, Y.; Shi, J. Prediction of new targets and mechanisms for quercetin in the treatment of pancreatic cancer, colon cancer, and rectal cancer. Food Funct. 2019, 10, 5339–5349. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Romani, A.; Galardi, C.; Pinelli, P.; Giaccherini, C.; Vincieri, F.F. Polyphenolic content in olive oil waste waters and related olive samples. J. Agric. Food Chem. 2001, 49, 3509–3514. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.N.; Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Effects of Electric Fields on Protein Unfolding and Aggregation: Influence on Edible Films Formation. Biomacromolecules 2010, 11, 2912–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira-Santos, P.; Genisheva, Z.; Pereira, R.N.; Teixeira, J.A.; Rocha, C.M.R. Moderate Electric Fields as a Potential Tool for Sustainable Recovery of Phenolic Compounds from Pinus pinaster Bark. ACS Sustain. Chem. Eng. 2019, 7, 8816–8826. [Google Scholar] [CrossRef] [Green Version]
- Rocha, C.M.R.; Genisheva, Z.; Ferreira-Santos, P.; Rodrigues, R.; Vicente, A.A.; Teixeira, J.A.; Pereira, R.N. Electric field-based technologies for valorization of bioresources. Bioresour. Technol. 2018, 254, 325–339. [Google Scholar] [CrossRef] [Green Version]
- Duncan, M.; Moschopoulou, E.; Herrington, E.; Deane, J.; Roylance, R.; Jones, L.; Bourke, L.; Morgan, A.; Chalder, T.; Thaha, M.A.; et al. Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors. BMJ Open 2017, 7, e015860. [Google Scholar] [CrossRef]
- Sluiter, A.; Ruiz, A.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008; Issue Date 7/17/2005-42619.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass—NREL/TP-510-42618: Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008; Issue Date 7/17/2005.
- Mussatto, S.I.; Roberto, I.C. Chemical characterization and liberation of pentose sugars from brewer’s spent grain. J. Chem. Technol. Biotechnol. 2006, 81, 268–274. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Selection of the Solvent and Extraction Conditions for Maximum Recovery of Antioxidant Phenolic Compounds from Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 1322–1332. [Google Scholar] [CrossRef] [Green Version]
- Jesus, M.S.; Romaní, A.; Genisheva, Z.; Teixeira, J.A.; Domingues, L. Integral valorization of vine pruning residue by sequential autohydrolysis stages. J. Clean. Prod. 2017, 168, 74–86. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydr. Polym. 2015, 127, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Library of Medicine. PubChem Open Chemistry Database. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 19 January 2022).
- Calculation of Molecular Properties and Bioactivity. Available online: http://www.molinspiration.com/ (accessed on 12 October 2021).
- Velderrain-Rodríguez, G.R.; Quero, J.; Osada, J.; Martín-Belloso, O.; Rodríguez-Yoldi, M.J. Phenolic-rich extracts from avocado fruit residues as functional food ingredients with antioxidant and antiproliferative properties. Biomolecules 2021, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- pkCSM Online Program. Available online: http://biosig.unimelb.edu.au/pkcsm/prediction (accessed on 12 January 2022).
- Uzarski, J.S.; DiVito, M.D.; Wertheim, J.A.; Miller, W.M. Essential design considerations for the resazurin reduction assay to noninvasively quantify cell expansion within perfused extracellular matrix scaffolds. Biomaterials 2017, 129, 163–175. [Google Scholar] [CrossRef]
- Sánchez-de-Diego, C.; Mármol, I.; Pérez, R.; Gascón, S.; Rodriguez-Yoldi, M.J.; Cerrada, E. The anticancer effect related to disturbances in redox balance on Caco-2 cells caused by an alkynyl gold(I) complex. J. Inorg. Biochem. 2017, 166, 108–121. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, T.B.; Oliveira, A.L.; Costa, C.; Nunes, J.; Vicente, A.A.; Pintado, M. Total and sustainable valorisation of olive pomace using a fractionation approach. Appl. Sci. 2020, 10, 6785. [Google Scholar] [CrossRef]
- Rafińska, K.; Pomastowski, P.; Rudnicka, J.; Krakowska, A.; Maruśka, A.; Narkute, M.; Buszewski, B. Effect of solvent and extraction technique on composition and biological activity of Lepidium sativum extracts. Food Chem. 2019, 289, 16–25. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and Sustainable Valorization of Bioactive Phenolic Compounds from Pinus By-Products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef]
- Nunes, M.A.; Palmeira, J.D.; Melo, D.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Alves, R.C.; Ferreira, H.; Oliveira, M.B.P.P. Chemical composition and antimicrobial activity of a new olive pomace functional ingredient. Pharmaceuticals 2021, 14, 913. [Google Scholar] [CrossRef]
- Čepo, D.V.; Radić, K.; Jurmanović, S.; Jug, M.; Rajković, M.G.; Pedisić, S.; Moslavac, T.; Albahari, P. Valorization of olive pomace-based nutraceuticals as antioxidants in chemical, food, and biological models. Molecules 2018, 23, 2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cioffi, G.; Pesca, M.S.; De Caprariis, P.; Braca, A.; Severino, L.; De Tommasi, N. Phenolic compounds in olive oil and olive pomace from Cilento (Campania, Italy) and their antioxidant activity. Food Chem. 2010, 121, 105–111. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Casazza, A.A.; Perego, P. Valorization of olive oil solid waste using high pressure-high temperature reactor. Food Chem. 2011, 128, 704–710. [Google Scholar] [CrossRef]
- Firdaus, M.L.; Fitriani, I.; Wyantuti, S.; Hartati, Y.W.; Khaydarov, R.; Mcalister, J.A.; Obata, H.; Gamo, T. Colorimetric detection of mercury(II) ion in aqueous solution using silver nanoparticles. Anal. Sci. 2017, 33, 831–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducom, G.; Gautier, M.; Pietraccini, M.; Tagutchou, J.P.; Lebouil, D.; Gourdon, R. Comparative analyses of three olive mill solid residues from different countries and processes for energy recovery by gasification. Renew. Energy 2020, 145, 180–189. [Google Scholar] [CrossRef]
- Velásquez-Jiménez, D.; Corella-Salazar, D.A.; Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Montiel-Herrera, M.; Salazar-López, N.J.; Rodrigo-Garcia, J.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Phenolic compounds that cross the blood–brain barrier exert positive health effects as central nervous system antioxidants. Food Funct. 2021, 12, 10356–10369. [Google Scholar] [CrossRef]
- Parveen, N.; Ali, S.A.; Ali, A.S. Insights Into the explication of potent tyrosinase inhibitors with reference to computational studies. Lett. Drug Des. Discov. 2019, 16, 1182–1193. [Google Scholar] [CrossRef]
- Freitas, T.S.; Xavier, J.C.; Pereira, R.L.S.; Rocha, J.E.; Campina, F.F.; de Araújo Neto, J.B.; Silva, M.M.C.; Barbosa, C.R.S.; Marinho, E.S.; Nogueira, C.E.S.; et al. In Vitro and in silico studies of chalcones derived from natural acetophenone inhibitors of NorA and MepA multidrug efflux pumps in Staphylococcus aureus. Microb. Pathog. 2021, 161, 105286. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Torres-Moreno, H.; Villegas-Ochoa, M.A.; Ayala-Zavala, J.F.; Robles-Zepeda, R.E.; Wall-Medrano, A.; González-Aguilar, G.A. Gallic acid content and an antioxidant mechanism are responsible for the antiproliferative activity of “Ataulfo” mango peel on LS180 cells. Molecules 2018, 23, 695. [Google Scholar] [CrossRef] [Green Version]
- Abbaszadeh, H.; Keikhaei, B.; Mottaghi, S. A review of molecular mechanisms involved in anticancer and antiangiogenic effects of natural polyphenolic compounds. Phyther. Res. 2019, 33, 2002–2014. [Google Scholar] [CrossRef]
- Curti, V.; Di Lorenzo, A.; Dacrema, M.; Xiao, J.; Nabavi, S.M.; Daglia, M. In Vitro polyphenol effects on apoptosis: An update of literature data. Semin. Cancer Biol. 2017, 46, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Polerà, N.; Badolato, M.; Filomena, P.; Carullo, G.; Aiello, F. Quercetin and its natural sources in wound healing management. Curr. Med. Chem. 2019, 26, 5825–5848. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Cappello, A.R.; Frattaruolo, L.; Badolato, M.; Armentano, B.; Aiello, F. Quercetin and derivatives: Useful tools in inflammation and pain management. Future Med. Chem. 2017, 9, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Rady, H.M.; Hemmaid, K.Z.; Esmaeil, N.N.; Eid, M.M.; Elshat, A.A. Sidr Kashmiry honey and its fractions induced apoptosis in hepatocellular carcinoma in vitro. Med. J. Nutr. Metab. 2018, 11, 343–351. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P. Potential roles of berries in the prevention of breast cancer progression. J. Berry Res. 2018, 8, 307–323. [Google Scholar] [CrossRef]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; La Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the main polyphenol of Olea europaea leaf extract, has an anti-cancer effect on human BRAF melanoma cells and potentiates the cytotoxicity of current chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunil, C.; Xu, B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef] [PubMed]
- Quero, J.; Jiménez-Moreno, N.; Esparza, I.; Osada, J.; Cerrada, E.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M.J. Grape stem extracts with potential anticancer and antioxidant properties. Antioxidants 2021, 10, 243. [Google Scholar] [CrossRef]
- Bai, L.; Wang, S. Targeting apoptosis pathways for new cancer therapeutics. Annu. Rev. Med. 2014, 65, 139–155. [Google Scholar] [CrossRef]
- Tanikawa, C.; Zhang, Y.Z.; Yamamoto, R.; Tsuda, Y.; Tanaka, M.; Funauchi, Y.; Mori, J.; Imoto, S.; Yamaguchi, R.; Nakamura, Y.; et al. The Transcriptional Landscape of p53 Signalling Pathway. EBioMedicine 2017, 20, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.; Chinkwo, K.; Santhakumar, A.; Johnson, S.; Blanchard, C. Apoptosis induction pathway in human colorectal cancer cell line SW480 exposed to cereal phenolic extracts. Molecules 2019, 24, 2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvajal, L.A.; Manfredi, J.J. Another fork in the road—Life or death decisions by the tumour suppressor p53. EMBO Rep. 2013, 14, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Fujiwara, T.; Kadowaki, Y.; Fukazawa, T.; Waku, T.; Itoshima, T.; Yamatsuji, T.; Nishizaki, M.; Roth, J.A.; Tanaka, N. Overexpression of the wild-type p53 gene inhibits NF-κB activity and synergizes with aspirin to induce apoptosis in human colon cancer cells. Oncogene 2000, 19, 726–736. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Darvin, P.; Lim, E.J.; Joung, Y.H.; Hong, D.Y.; Park, E.U.; Park, S.H.; Choi, S.K.; Moon, E.S.; Cho, B.W.; et al. Hwanggeumchal sorghum induces cell cycle arrest, and suppresses tumor growth and metastasis through jak2/stat pathways in breast cancer xenografts. PLoS ONE 2012, 7, e40531. [Google Scholar] [CrossRef]
- Cipolletti, M.; Fernandez, V.S.; Montalesi, E.; Marino, M.; Fiocchetti, M. Beyond the antioxidant activity of dietary polyphenols in cancer: The modulation of estrogen receptors (ERs) signaling. Int. J. Mol. Sci. 2018, 19, 2624. [Google Scholar] [CrossRef] [Green Version]
- Roy, P.; George, J.; Srivastava, S.; Tyagi, S.; Shukla, Y. Inhibitory effects of tea polyphenols by targeting cyclooxygenase-2 through regulation of nuclear factor kappa B, Akt and p53 in rat mammary tumors. Investig. New Drugs 2011, 29, 225–231. [Google Scholar] [CrossRef]
- Garrido-Armas, M.; Corona, J.C.; Escobar, M.L.; Torres, L.; Ordóñez-Romero, F.; Hernández-Hernández, A.; Arenas-Huertero, F. Paraptosis in human glioblastoma cell line induced by curcumin. Toxicol. Vitr. 2018, 51, 63–73. [Google Scholar] [CrossRef]
- Suvorova, I.I.; Knyazeva, A.R.; Pospelov, V.A. Resveratrol-induced p53 activation is associated with autophagy in mouse embryonic stem cells. Biochem. Biophys. Res. Commun. 2018, 503, 2180–2185. [Google Scholar] [CrossRef]
- Vitkeviciene, A.; Baksiene, S.; Borutinskaite, V.; Navakauskiene, R. Epigallocatechin-3-gallate and BIX-01294 have different impact on epigenetics and senescence modulation in acute and chronic myeloid leukemia cells. Eur. J. Pharmacol. 2018, 838, 32–40. [Google Scholar] [CrossRef]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Rosillo, M.A.; De La Lastra, C.A. Oleuropein, a secoiridoid derived from olive tree, inhibits the proliferation of human colorectal cancer cell through downregulation of HIF-1α. Nutr. Cancer 2013, 65, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef] [PubMed]
- Arul, D.; Subramanian, P. Naringenin (Citrus Flavonone) induces growth inhibition, cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Pathol. Oncol. Res. 2013, 19, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.C.; Yang, J.S.; Lu, H.F.; Ip, S.W.; Lo, C.; Wu, C.C.; Lin, J.P.; Tang, N.Y.; Chung, J.G.; Chou, M.J.; et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch. Pharm. Res. 2010, 33, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Manda, G.; Isvoranu, G.; Comanescu, M.V.; Manea, A.; Debelec Butuner, B.; Korkmaz, K.S. The redox biology network in cancer pathophysiology and therapeutics. Redox Biol. 2015, 5, 347–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budanov, A.V. The Role of Tumor Suppressor p53 in the Antioxidant Defense and Metabolism. In Mutant p53 and MDM2 in Cancer; Deb, S.P., Deb, S., Eds.; Springer: Richmond, VA, USA, 2014; Volume 85, pp. 337–358. ISBN 978-94-017-9210-3. [Google Scholar]
- Gump, J.M.; Thorburn, A. Autophagy and apoptosis: What is the connection? Trends Cell Biol. 2011, 21, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Enayat, S.; Ceyhan, M.Ş.; Başaran, A.A.; Gürsel, M.; Banerjee, S. Anticarcinogenic effects of the ethanolic extract of salix aegyptiaca in colon cancer cells: Involvement of Akt/PKB and MAPK pathways. Nutr. Cancer 2013, 65, 1045–1058. [Google Scholar] [CrossRef]
- Monga, J.; Pandit, S.; Chauhan, R.S.; Chauhan, C.S.; Chauhan, S.S.; Sharma, M. Growth Inhibition and Apoptosis Induction by (+)-Cyanidan-3-ol in Hepatocellular Carcinoma. PLoS ONE 2013, 8, 1–19. [Google Scholar] [CrossRef] [Green Version]
- De Bruno, A.; Romeo, R.; Fedele, F.L.; Sicari, A.; Piscopo, A.; Poiana, M. Antioxidant activity shown by olive pomace extracts. J. Environ. Sci. Health-Part B Pestic. Food Contam. Agric. Wastes 2018, 53, 526–533. [Google Scholar] [CrossRef]
- Rathi, M.H.; Turki, R.H. Total Phenolic Contents and Antioxidant Activities of Olive (Olea europaea L.) Pomace and Their Ingredients. J. Al-Nahrain Univ. Sci. 2018, 21, 106–111. [Google Scholar] [CrossRef]
- Sinrod, A.J.G.; Avena-Bustillos, R.J.; Olson, D.A.; Crawford, L.M.; Wang, S.C.; McHugh, T.H. Phenolics and Antioxidant Capacity of Pitted Olive Pomace Affected by Three Drying Technologies. J. Food Sci. 2019, 84, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Ultrasound increases the aqueous extraction of phenolic compounds with high antioxidant activity from olive pomace. LWT-Food Sci. Technol. 2018, 89, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Almeer, R.S.; Mahmoud, S.M.; Amin, H.K.; Abdel Moneim, A.E. Ziziphus spina-christi fruit extract suppresses oxidative stress and p38 MAPK expression in ulcerative colitis in rats via induction of Nrf2 and HO-1 expression. Food Chem. Toxicol. 2018, 115, 49–62. [Google Scholar] [CrossRef]
- Kumar, V.L.; Pandey, A.; Verma, S.; Das, P. Protection afforded by methanol extract of Calotropis procera latex in experimental model of colitis is mediated through inhibition of oxidative stress and pro-inflammatory signaling. Biomed. Pharmacother. 2019, 109, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Chalouati, H.; Boutet, E.; Metais, B.; Fouche, E.; Ben Sâad, M.M.; Gamet-Payrastre, L. DNA damage and oxidative stress induced at low doses by the fungicide hexachlorobenzene in human intestinal Caco-2 cells. Toxicol. Mech. Methods 2015, 25, 448–458. [Google Scholar]
- Lee, S.I.; Kang, K.S. N-acetylcysteine modulates lipopolysaccharide-induced intestinal dysfunction. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Catanzaro, D.; Rancan, S.; Orso, G.; Dall’acqua, S.; Brun, P.; Giron, M.C.; Carrara, M.; Castagliuolo, I.; Ragazzi, E.; Caparrotta, L.; et al. Boswellia serrata preserves intestinal epithelial barrier from oxidative and inflammatory damage. PLoS ONE 2015, 10, e0125375. [Google Scholar] [CrossRef] [Green Version]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Andreu, V.; Larrea, A.; Rodriguez-Fernandez, P.; Alfaro, S.; Gracia, B.; Lucía, A.; Usón, L.; Gomez, A.-C.; Mendoza, G.; Lacoma, A.; et al. Matryoshka-type gastro-resistant microparticles for the oral treatment of Mycobacterium tuberculosis. Nanomedicine 2019, 14, 707–726. [Google Scholar] [CrossRef] [Green Version]








| Proximate Composition (g/100 g Dry OP) | Mineral Element (mg/Kg Dry OP) | ||
|---|---|---|---|
| Cellulose a | 16.16 ± 0.78 | Potassium | 2798.44 ± 29.64 |
| Hemicellulose | 18.96 ± 1.79 | Calcium | 352.03 ± 23.01 |
| Xylose | 15.32 ± 0.45 | Magnesium | 145.41 ± 16.54 |
| Arabinose | 3.64 ± 0.20 | Iron | 12.24 ± 2.71 |
| Acetyl group | 3.75 ± 0.73 | Sodium | 16. 42 ± 0.67 |
| Lignin | 31.69 ± 1.40 | Aluminum | 16.93 ± 2.08 |
| Insoluble | 20.47 ± 1.31 | Manganese | 1.56 ± 0.22 |
| Soluble | 11.22 ± 0.13 | Zinc | 2.92 ± 0.36 |
| Protein | 5.66 ± 0.31 | Copper | 2.73 ± 0.29 |
| Fat | 12.06 ± 0.79 | Boron | 4.02 ± 0.52 |
| Ashes | 4.55 ± 0.22 | Barium | 0.18 ± 0.03 |
| Total extractives b | 25.23 ± 1.88 | ||
| Method | Solvent | TPC (mg GAE/g OP) | Antioxidant Activity | Yield (%) | |
|---|---|---|---|---|---|
| DPPH * (µmol TE/g OP) | FRAP (µmol Fe(II)/g OP) | ||||
| OH | H2O | 12.08 ± 1.52 a | 3.23 ± 0.10 b | 80.41 ± 1.12 a | 28.54 ± 0.14 a |
| EtOH 50% | 17.67 ± 3.12 b | 3.82 ± 0.04 a | 150.16 ± 9.29 b | 27.39 ± 1.54 ac | |
| CH | H2O | 12.24 ± 0.88 a | 3.36 ± 0.03 b | 80.45 ± 2.45 a | 24.70 ± 0.27 b |
| EtOH 50% | 16.89 ± 0.76 b | 3.56 ± 0.05 c | 130.34 ± 2.39 c | 25.60 ± 0.19 bc | |
| Phenolic Compound | OH | CH | ||
|---|---|---|---|---|
| H2O | EtOH 50% | H2O | EtOH 50% | |
| Hydroxytyrosol and tyrosol derivatives | ||||
| Hydroxytyrosol | 28.71 ± 0.42 a | 33.36 ± 0.57 b | 31.20 ± 0.29 c | 33.49 ± 0.40 b |
| Tyrosol | 10.63 ± 0.06 a | 21.09 ± 0.12 b | 11.13 ± 0.15 a | 11.09 ± 0.99 a |
| Oleuropein | n.d. | 254.38 ± 13.24 a | n.d. | 369.05 ± 23.00 b |
| Phenolic acids | ||||
| Caffeic acid | n.d. | 2.59 ± 0.47 a | n.d. | 2.27 ± 0.35 a |
| Cinnamic acid | 18.45 ± 0.95 a | 19.99 ± 0.46 b | n.d. | n.d. |
| p-Coumaric acid | 44.99 ± 3.01 a | 48.41 ± 2.84 a | 20.91 ± 0.91 b | 48.76 ± 1.49 a |
| o-Coumaric acid | 23.26 ± 1.24 a | 69.81 ± 1.14 b | 25.45 ± 0.41 a | 48.93 ± 1.02 c |
| Ferulic acid | 22.31 ± 1.38 a | 13.83 ± 1.28 b | 23.60 ± 1.22 a | 22.40 ± 0.51 a |
| Vanillic acid | 39.51 ± 0.48 a | 72.77 ± 1.11 b | 43.96 ± 1.79 c | 59.41 ± 0.50 d |
| 3,4-Dihidroxibenzoic acid | 14.20 ± 0.28 a | 17.61 ± 0.94 b | 15.60 ± 0.37 ac | 16.61 ± 0.39 bc |
| Syringic acid | n.d. | 42.98 ± 3.74 a | n.d. | n.d. |
| Ellagic acid | 142.93 ± 4.49 a | 147.82 ± 3.40 a | 162.01 ± 0.61 b | 148.40 ± 3.85 a |
| Homovanillic acid | 103.45 ± 6.24 a | 75.54 ± 0.56 b | 118.62 ± 5.56 c | n.d. |
| Rosmarinic acid | 22.05 ± 0.80 a | 62.79 ± 2.27 b | n.d. | n.d. |
| Flavonoids | ||||
| Apigenin | n.d. | 43.13 ± 1.28 a | n.d. | 42.48 ± 1.31 a |
| Rutin | 21.33 ± 0.62 a | 31.46 ± 2.68 b | n.d. | n.d. |
| Taxifolin | 83.10 ± 2.23 a | 94.44 ± 4.01 b | 85.82 ± 3.40 a | 89.03 ± 2.42 a |
| Naringenin | 125.11 ± 0.56 a | 247.42 ± 10.19 b | 99.96 ± 4.62 c | 137.67 ± 10.14 a |
| Hesperidin | 137.30 ± 5.22 a | 93.15 ± 4.24 b | 62.77 ± 1.46 c | 85.75 ± 2.44 b |
| Quercetin | 168.62 ± 15.47 a | 134.94 ± 0.89 b | 206.01 ± 6.29 c | 134.14 ± 2.85 b |
| Catechin | 63.15 ± 2.39 a | 77.68 ± 1.06 b | 55.29 ± 1.58 c | 49.04 ± 0.78 d |
| Stilbene | ||||
| Resveratrol | 6.68 ± 1.02 a | 10.34 ± 0.29 b | n.d. | n.d. |
| Total | 1075.88 | 1615.54 | 962.23 | 1298.53 |
| Phenolic Compound | MW | TPSA | Log P | No. atoms | Hydrogen Bonds Acceptors | Hydrogen Bonds Donors | Rotatable Bonds | Molecular Volume (Å3) | Violations to LIRF | % ABS | Log Papp |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxytyrosol and tyrosol derivatives | |||||||||||
| Hydroxytyrosol | 154.16 | 60.68 | 0.52 | 11 | 3 | 3 | 2 | 141.70 | 0 | 88.07 | 1.09 |
| Tyrosol | 138.17 | 40.46 | 1.00 | 10 | 2 | 2 | 2 | 133.68 | 0 | 95.04 | 1.69 |
| Oleuropein | 540.52 | 201.68 | −0.36 | 38 | 13 | 6 | 11 | 466.31 | 3 | 39.42 | 0.06 |
| Phenolic acids | |||||||||||
| Caffeic acid | 180.16 | 77.75 | 0.94 | 13 | 4 | 3 | 2 | 154.50 | 0 | 82.18 | 0.63 |
| Cinnamic acid | 148.16 | 37.30 | 1.91 | 11 | 2 | 1 | 2 | 138.46 | 0 | 96,13 | 1.71 |
| p-Coumaric acid | 164.16 | 57.53 | 1.43 | 12 | 3 | 2 | 2 | 146.48 | 0 | 89.15 | 1.21 |
| o-coumaric acid | 164.16 | 57.53 | 1.67 | 12 | 3 | 2 | 2 | 146.48 | 0 | 89.15 | 1.21 |
| Ferulic acid | 194.19 | 66.76 | 1.25 | 14 | 4 | 2 | 3 | 172.03 | 0 | 85.97 | 0.17 |
| Vanillic acid | 168.15 | 66.76 | 1.19 | 12 | 4 | 2 | 2 | 144.61 | 0 | 85.97 | 0.33 |
| 3,4-Dihidroxibenzoic acid | 154.12 | 77.75 | 0.88 | 11 | 4 | 3 | 1 | 127.08 | 0 | 82.17 | 0.49 |
| Syringic acid | 198.17 | 76 | 1.20 | 14 | 5 | 2 | 3 | 170.15 | 0 | 82.78 | 0.49 |
| Ellagic acid | 302.19 | 141.33 | 0.94 | 22 | 8 | 4 | 0 | 221.78 | 0 | 60.24 | 0.33 |
| Homovanillic acid | 182.18 | 66.76 | 0.70 | 13 | 4 | 2 | 3 | 161.41 | 0 | 85.96 | 0.26 |
| Rosmarinic acid | 360.32 | 144.52 | 1.63 | 26 | 8 | 5 | 7 | 303.54 | 0 | 59.14 | −0.93 |
| Flavonoids | |||||||||||
| Apigenin | 270.24 | 90.89 | 2.46 | 20 | 5 | 3 | 1 | 224.05 | 0 | 90.89 | 1.00 |
| Rutin | 610.52 | 269.43 | −1.06 | 43 | 16 | 10 | 6 | 496.07 | 3 | 16.04 | −0.94 |
| Taxifolin | 304.25 | 127.44 | 0.71 | 22 | 7 | 5 | 1 | 246.32 | 0 | 65.03 | 0.92 |
| Naringenin | 272.26 | 86.99 | 2.12 | 20 | 5 | 3 | 1 | 230.26 | 0 | 78.99 | 1.02 |
| Hesperidin | 610.57 | 234.30 | −0.55 | 43 | 15 | 8 | 7 | 511.79 | 3 | 28.16 | 0.50 |
| Quercetin | 302.24 | 131.35 | 1.68 | 22 | 11 | 7 | 1 | 240.08 | 0 | 63.68 | −0.22 |
| Catechin | 290.27 | 110.37 | 1.37 | 21 | 6 | 5 | 1 | 244.14 | 0 | 70.92 | −0.28 |
| Stilben | |||||||||||
| Resveratrol | 228.25 | 60.68 | 2.99 | 17 | 3 | 3 | 2 | 206.92 | 0 | 88.06 | 1.17 |
| Extract | Caco-2 Undifferentiated | Caco-2 Differentiated |
|---|---|---|
| CH-H2O | 2256.1 ± 237.56 a | >5000 a |
| CH-EtOH | 747.99 ± 140.97 b | >5000 a |
| OH-H2O | 2817.10 ± 53.28 a | >5000 a |
| OH-EtOH | 692.32 ± 63.58 b | 4026 ± 274 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quero, J.; Ballesteros, L.F.; Ferreira-Santos, P.; Velderrain-Rodriguez, G.R.; Rocha, C.M.R.; Pereira, R.N.; Teixeira, J.A.; Martin-Belloso, O.; Osada, J.; Rodríguez-Yoldi, M.J. Unveiling the Antioxidant Therapeutic Functionality of Sustainable Olive Pomace Active Ingredients. Antioxidants 2022, 11, 828. https://doi.org/10.3390/antiox11050828
Quero J, Ballesteros LF, Ferreira-Santos P, Velderrain-Rodriguez GR, Rocha CMR, Pereira RN, Teixeira JA, Martin-Belloso O, Osada J, Rodríguez-Yoldi MJ. Unveiling the Antioxidant Therapeutic Functionality of Sustainable Olive Pomace Active Ingredients. Antioxidants. 2022; 11(5):828. https://doi.org/10.3390/antiox11050828
Chicago/Turabian StyleQuero, Javier, Lina F. Ballesteros, Pedro Ferreira-Santos, Gustavo R. Velderrain-Rodriguez, Cristina M. R. Rocha, Ricardo N. Pereira, José A. Teixeira, Olga Martin-Belloso, Jesús Osada, and María Jesús Rodríguez-Yoldi. 2022. "Unveiling the Antioxidant Therapeutic Functionality of Sustainable Olive Pomace Active Ingredients" Antioxidants 11, no. 5: 828. https://doi.org/10.3390/antiox11050828