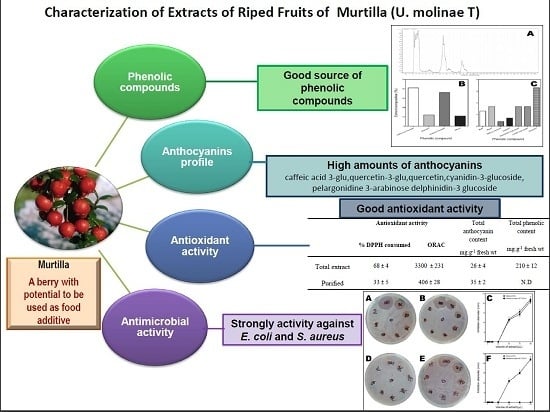
Isolation and Characterization of Phenolic Compounds and Anthocyanins from Murta (Ugni molinae Turcz.) Fruits. Assessment of Antioxidant and Antibacterial Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Phenolic Compounds of Murta Extracts


2.2. Identification and Quantification of Anthocyanins by HPLC/ESI-MS

| Peak No | Molucular Ion (m/z) | Fragment (m/z) | Compound | Proportion of each Crude Extract | Compound (%) Pure Extract |
|---|---|---|---|---|---|
| 1 | 465 | 303 | Delphinidin 3-O-glu | 40.0 | 73.0 |
| 2 | 479 | 371 | Petunidin 3-O-glu | 6.0 | 5.0 |
| 3 | 463 | 301 | Peonidin 3-O-glu | 0.7 | 3.0 |
| 4 | 463 | 331 | Malvidin 3-O-glu | 0.4 | 0.1 |
| 5 | 449 | 287 | Cyanidin 3-O-glu | 0.4 | 4.3 |
| 6 | 449 | 287 | Cyanidin 3-O-gal | 0.5 | 2.6 |
| 7 | 435 | 303 | Delphinidin 3-O-ara | 3.6 | 10.0 |
| 8 | 519 | 271 | Pelargonidin 3-O-ara | 49.0 | 1.0 |
| 9 | 419 | 287 | Cyanidin 3-O-ara | ND | 1.0 |
| 10 | 549 | 271 | Peonidin-malvidin 3-O-glu | 2.0 | 3.0 |

2.3. Antioxidant Capacity
| Antioxidant Activity | Total Anthocyanin (mg·g−1 fresh wt) | Total Phenolic (mg·g−1 fresh wt) | ||
|---|---|---|---|---|
| % DPPH | ORAC Value (µmol Trolox/g) | |||
| Total extract | 68 ± 4 | 3300 ± 231 | 26 ± 4 | 210 ± 12 |
| Purified extract | 33 ± 5 | 406 ± 28 | 35 ± 2 | - |
2.4. Antibacterial Activity
| Antibiotic | E. coli | S. typhi |
|---|---|---|
| Tetracycline | 28.3 ± 0.9 | 23.7 ± 0.4 |
| Clotrimazole | 11.0 ± 0.6 | 22.7 ± 0.7 |
| Gentamicin | 10.7 ± 0.3 | 24.3 ± 0.3 |
| Amikacin | 20.7 ± 0.3 | 24.0 ±.0.3 |
| Ceftriaxone | 17.7 ± 0.9 | 23.3 ± 0.7 |
| Cefuroxim | 20.7 ± 0.7 | 24.3 ± 0.3 |
| Cefotaxim | 19.3 ± 0.7 | 24.3 ± 0.3 |
| Ampicillin | 22.3 ± 0.9 | 22.7 ± 0.3 |
| Ciprofloxacin | 22.3 ± 0.9 | 23.7 ± 0.9 |
| Ampicillin/Sulbactam | 12.3 ± 0.7 | 23.7 ± 0.3 |
3. Experimental Section
3.1. Chemicals and Solvents
3.2. Plant Material
3.3. Extraction of Phenolic Compounds and Anthocyanins
3.4. Purification of Anthocyanins
3.5. Analysis of Phenolic Content
3.6. Chemical Identification of Anthocyanins
3.7. Analysis of Antioxidant Capacity (DPPH• and ORAC Assays)
- AUC = Area under curve in the presence of the tested extract sample, integrated between time zero and that corresponding to 80% of the probe consumption;
- AUC° = Area under curve of control (PGR or FL plus AAPH solution);
- AUCtrolox = Area under curve in the presence of Trolox;
- f = Dilution factor, equal to the ratio between the total volume of the working solution (target molecule plus AAPH, plus extract) and the added extract sample volume;
- [Trolox] = Trolox milimolar concentration.
3.8. Antibacterial Activity of Extracts
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paredes-López, O.; Cervantes-Ceja, M.; Vigna-Pérez, M.; Hernández-Pérez, T. Berries: Improving human health and healthy aging, and promoting quality life—A review. Plant Foods Hum. Nutr. 2010, 65, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Arancibia-Avila, P.; Toledo, F.; Werner, E.; Suhaj, M.; Leontowicz, H.; Leontowicz, M.; Martinez-Ayala, A.L.; Paśko, P.; Gorinstein, S. Partial characterization of a new kind of Chilean murtilla-like berries. Food Res. Int. 2011, 44, 2054–2062. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and antioxidant activity of calafate (Berberis microphylla) fruits and other native berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Wesely, E.G.; Zahir, H.M.I.; Selvan, N. In vivo and in vitro phytochemical and antibacterial efficacy of Baliospermum montanum (Wïlld.) Muell. Arg. Asian Pac. J. Trop. Med. 2010, 3, 894–897. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Ahmad, M.; Mehjabeen; Jehan, N.; Ahmad, S.; Qayum, M.; Marwat, K.I. Antimicrobial screening of selected flora of Pakistan. Arch. Biol. Sci. 2011, 63, 691–695. [Google Scholar] [CrossRef]
- Machiex, J.-J.; Fleuriet, A.; Billot, J. Importance and roles of phenolic compounds in fruits. In Fruit Phenolics; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Cao, G.; Sofic, E.; Prior, R. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Díaz-Mula, H.M.; Valero, D. Antioxidant compounds in fruits and vegetables and changes during postharvest storage and processing. Stewart Posthar. Rev. 2011, 7, 1–10. [Google Scholar] [CrossRef]
- Pazmiño-Durán, E.A.; Giusti, M.M.; Wrolstad, R.E.; Gloria, M.B.A. Anthocyanins from Oxalis triangularis as potential food colorants. Food Chem. 2001, 75, 211–216. [Google Scholar] [CrossRef]
- Longo, L.; Vasapollo, G. Extraction and Identification of anthocyanins from Smilax aspera L. berries. Food Chem. 2006, 94, 226–231. [Google Scholar] [CrossRef]
- Tsai, P.J.; McIntosh, J.; Pearce, P.; Camden, B.; Jordan, B.R. Anthocyanins and antioxidants capacity in Roselle (Hibiscus sabdariffa L.) extract. Food Res. Int. 2002, 35, 351–356. [Google Scholar] [CrossRef]
- Prior, X.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radic. Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Muñoz, C.; Godoy, I. Especie nativa con potencial como frutales arbustivos. Investig. Prog. Agropecu. Carillanca 1996, 5, 32–35. [Google Scholar]
- Montenegro, G. Nuestra Flora Útil; Ediciones Universidad Católica de Chile: Santiago, Chile, 2000. [Google Scholar]
- Pastenes, C.; Santa-María, E.; Infante, R.; Franck, N. Domestication of the Chilean guava (Ugni molinae Turcz.), a forest under storey shrub, must consider light intensity. Sci. Hortic. 2003, 98, 71–84. [Google Scholar] [CrossRef]
- Scheuermann, E.; Seguel, I.; Montenegro, A.; Bustos, R.O.; Hormazabal, E.; Quiroz, A. Evolution of aroma compounds of murtilla fruits (Ugni molinae Turcz) during storage. J. Sci. Food Agric. 2008, 88, 485–492. [Google Scholar] [CrossRef]
- Taboada, E.; Fisher, P.; Jara, R.; Zuñiga, E.; Gidekel, M.; Cabrera, J.C.; Pereira, E.; Gutiérrez-Moraga, A.; Villalonga, R.; Cabrera, G. Isolation and characterisation of pectic substances from murta (Ugni molinae Turcz) fruits. Food Chem. 2010, 123, 669–678. [Google Scholar] [CrossRef]
- Avello, M.; Pastene, E. Actividad antioxidante de infusos de Ugni molinae Turcz. (“Murtilla”). Blacpma 2005, 4, 33–39. [Google Scholar]
- Rubilar, M.; Pinelo, M.; Ihl, M.; Scheuermann, E.; Sineiro, J.; Nuñez, M.J. Murta leaves (Ugni molinae Turcz) as a source of antioxidant phenols. J. Agric. Food Chem. 2006, 54, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Shene, C.; Reyes, A.; Villarroel, M.; Sineiro, J.; Pinelo, M.; Rubilar, M. Plant location and extraction procedure strongly alter the antimicrobial activity of murta extracts. Eur. Food Res. Technol. 2009, 228, 467–475. [Google Scholar] [CrossRef]
- Rubilar, M.; Jara, C.; Poo, P.; Acevedo, F.; Gutierrez, C.; Sineiro, J.; Shene, C. Extracts of Maqui (Aristotelia chilensis) and Murta (Ugni molinae Turcz.): Sources of Antioxidant Compounds and α-Glucosidase/α-Amylase Inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Nishimura, H.; Morota, T.; Chin, M.; Mitsuhashi, H.; Komatso, Y.; Maruyama, H.; Tu, G.R.; Wei, H.; Xiong, Y.L. Immunosuppressive principle of Rehmannia glutinosa var. hueichingensis. Planta Med. 1989, 55, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073–2085. [Google Scholar]
- Formica, J.V.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Walsky, R.L.; Gaman, E.A.; Obach, R.S. Examination of 209 drugs for inhibition of cytochrome P450 2C8. J. Clin. Pharmacol. 2005, 45, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Otake, Y.; Nolan, A.L.; Walle, U.K.; Walle, T. Quercetin and resveratrol potently reduce estrogen sulfotransferase activity in normal human mammary epithelial cells. J. Steroid Biochem. Mol. Biol. 2000, 63, 265–270. [Google Scholar] [CrossRef]
- Huang, S.L.; Hsu, C.L.; Yen, G.C. Growth inhibitory effect of quercetin on SW 872 human liposarcoma cells. Life Sci. 2006, 79, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; van Trijp, J.M.; Buysman, M.N.; van der Gaag, M.S.; Mengelers, M.J.; de Vries, J.H.; Katan, M.B. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997, 418, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Leighton, T.; Ginther, C.; Fluss, L.; Harter, W.K.; Cansado, J.; Notario, V. Molecular Characterization of Quercetin and Quercetin Glycosides in Allium Vegetables. In Phenolic Compounds in Food and Their Effects on Health II; American Chemical Society: Washington, DC, USA, 1992; pp. 220–238. [Google Scholar]
- Yang, R.-Y.; Lin, S.; Kuo, G. Content and distribution of flavonoids among 91 edible plant species. Asia Pac. J. Clin. Nutr. 2008, 17, 275–279. [Google Scholar] [PubMed]
- Goet, R.K.; Pandey, V.B.; Dwivedi, S.P.D.; Rao, Y.V. Antiinflammatory and antiulcer effects of kaempferol, a flavone, isolated from Rhamnus procumbens. Indian J. Exp. Biol. 1988, 26, 121–124. [Google Scholar] [PubMed]
- Wilhelm, K.P.; Biel, S.; Siegers, C.P. Role of flavonoids in controlling the phototoxicity of Hypericum perforatum extract. Phytomedicine 2001, 8, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Ashida, H.; Danno, G. Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Biosci. Biotechnol. Biochem. 2002, 66, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- De Medina, F.S.; Vera, B.; Galvez, J.; Zarzuelo, A. Effect of quercitrin on the early stages of hapten induced colonic inflammation in the rat. Life Sci. 2001, 70, 3097–3108. [Google Scholar] [CrossRef]
- Pati, S.; Losito, I.; Gambacorta, G.; La Notte, E.; Palmisano, F.; Zambonin, P.G. Simultaneous separation and identification of oligomeric procyanidins and anthocyanin-derived pigments in raw red wine by HPLC-UV-ESI-MSn. J. Mass Spectrom. 2006, 41, 861–871. [Google Scholar] [CrossRef] [PubMed]
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Aguirre, M.C.; Delporte, C.; Backhouse, N.; Erazo, S.; Letelier, M.E.; Cassels, B.K.; Silva, X.; Alegría, S.; Negrete, R. Topical anti-inflammatory activity of 2a-hydroxy pentacyclic triterpene acids from the leaves of Ugni molinae. Bioorganic Med. Chem. 2006, 14, 5673–5677. [Google Scholar] [CrossRef]
- Delporte, C.; Backhouse, N.; Inostroza, V.; Aguirre, M.C.; Peredo, N.; Silva, X. Analgesic activity of Ugni molinae (murtilla) in mice models of acute pain. J. Ethnopharmacol. 2007, 112, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Capecka, E.; Mareczek, A.; Leja, M. Antioxidant activity of fresh and dry herbs of some Lamiaceae species. Food Chem. 2005, 93, 223–226. [Google Scholar] [CrossRef]
- Lopez, M.; Martinez, F.; Del Valle, C.; Ferrit, M.; Luque, R. Study of phenolic compounds as natural antioxidants by a fluorescence method. Talanta 2005, 6, 609–616. [Google Scholar]
- Aberoumand, A.; Deokule, S.S. Comparison of Phenolic Compounds of Some Edible Plants of Iran and India. Pak. J. Nutr. 2008, 7, 582–585. [Google Scholar] [CrossRef]
- Nascimento, P.L.A.; Nascimento, T.C.E.S.; Ramos, N.S.M.; Silva, G.R.; Galindo Gomes, J.E.; Falcão, R.E.A.; Moreira, K.A.; Porto, A.L.F.; Silva, T.M.S. Quantification, antioxidant and antimicrobial activity of phenolics isolated from different extracts of Capsicum frutescens (Pimenta Malagueta). Molecules 2014, 19, 5434–5447. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Chen, C.-T.; Sciarappa, W.; Wang, C.Y.; Camp, M.J. Fruit Quality, Antioxidant Capacity, and Flavonoid Content of Organically and Conventionally Grown Blueberries. J. Agric. Food Chem. 2008, 56, 5788–5794. [Google Scholar] [CrossRef] [PubMed]
- Turgis, M.; Borsa, J.; Millette, M.; Salmieri, S.; Lacroix, M. Effect of selected plant essential oils or their constituents and modified atmosphere packaging on the radio sensitivity of Escherichia coli O157:H7 and Salmonella Typhi in ground beef. J. Food Prot. 2008, 71, 516–521. [Google Scholar] [PubMed]
- Avello, M.; Valdivia, R.; Sanzana, R.; Mondaca, M.A.; Mennickent, S.; Aeschlimann, M.; Bittner, M.; Becerra, J. Extractos antioxidantes y antimicrobianos de Aristotelia chilensis y Ugni molinae y sus aplicaciones como preservantes en productos cosméticos. Bol. Latinoam. Caribe Plantas Med. Aromát. 2009, 8, 479–486. [Google Scholar]
- Mølgaard, P.; Hollera, J.G.; Asarb, B.; Libernaa, I.; Rosenbæka, L.B.; Jebjerga, C.P.; Jørgensen, L.; Lauritzen, J.; Guzman, A.; Adsersen, A.; et al. Antimicrobial evaluation of Huilliche plant medicine used to treat wounds. J. Ethnopharmacol. 2011, 138, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Venkata, S.P.; Murali, M.C.; Prameela, K.; Sravani, R.; Raju, B.A. Screening of Antimicrobial and Antioxidant Potentials of Acacia caesia, Dillenia pentagyna and Buchanania lanzan from Maredumilli Forest of India. J. Pharm. Res. 2012, 5, 1734–1738. [Google Scholar]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. IN. Extraction and determination of total anthocyanin in Cranberries. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Bravo, L.; Goya, L.; Lucumberri, E. LC/MS characterization of phenolic constituents of mate (Ilex paraguariensis, St. Hil.) and its antioxidant activity compared to commonly consumed beverages. Food Res. Int. 2007, 40, 393–405. [Google Scholar] [CrossRef]
- Qin, C.G.; Li, Y.; Zhang, R.J.; Niu, W.N.; Ding, Y. Separation and elucidation of anthocyanins in fruit of mockstrawberry (Duchesnea indica Focke). Nat. Product Res. 2009, 23, 1589–1598. [Google Scholar] [CrossRef]
- Shyu, Y.-S.; Lin, J.-T.; Chang, Y.-T.; Chiang, C.-J.; Yang, D.-J. Evaluation of antioxidant ability of ethanolic extract from dill (Anethum graveolens L.) flower. Food Chem. 2009, 115, 515–521. [Google Scholar] [CrossRef]
- Alarcón, E.; Campos, A.M.; Edwards, A.M.; Lissi, E.; López-Alarcón, C. Antioxidant capacity of herbal infusions and tea extracts: A comparison of ORAC-fluorescein and ORAC-pyrogallol red methodologies. Food Chem. 2008, 107, 1114–1122. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junqueira-Gonçalves, M.P.; Yáñez, L.; Morales, C.; Navarro, M.; A. Contreras, R.; Zúñiga, G.E. Isolation and Characterization of Phenolic Compounds and Anthocyanins from Murta (Ugni molinae Turcz.) Fruits. Assessment of Antioxidant and Antibacterial Activity. Molecules 2015, 20, 5698-5713. https://doi.org/10.3390/molecules20045698
Junqueira-Gonçalves MP, Yáñez L, Morales C, Navarro M, A. Contreras R, Zúñiga GE. Isolation and Characterization of Phenolic Compounds and Anthocyanins from Murta (Ugni molinae Turcz.) Fruits. Assessment of Antioxidant and Antibacterial Activity. Molecules. 2015; 20(4):5698-5713. https://doi.org/10.3390/molecules20045698
Chicago/Turabian StyleJunqueira-Gonçalves, Maria Paula, Lina Yáñez, Carolina Morales, Muriel Navarro, Rodrigo A. Contreras, and Gustavo E. Zúñiga. 2015. "Isolation and Characterization of Phenolic Compounds and Anthocyanins from Murta (Ugni molinae Turcz.) Fruits. Assessment of Antioxidant and Antibacterial Activity" Molecules 20, no. 4: 5698-5713. https://doi.org/10.3390/molecules20045698